Online Database of Chemicals from Around the World
| Suzhou Biosyntech Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 13921151340 +86 (512) 6300-1269 | |||
 |
sales2@biosyntech-suzhou.com sales@biosyntech-suzhou.com | |||
 |
QQ chat | |||
| Chemical distributor since 2019 | ||||
| chemBlink standard supplier since 2020 | ||||
| Classification | Organic raw materials >> Heterocyclic compound >> Pyrroles |
|---|---|
| Name | tert-Butyl (R)-3-((S)-1-((S)-4-benzyl-2-oxooxazolidin-3-yl)-3-(3-bromophenyl)-1-oxopropan-2-yl)pyrrolidine-1-carboxylate |
| Molecular Structure | 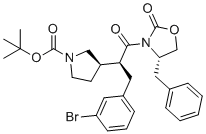 |
| Molecular Formula | C28H33BrN2O5 |
| Molecular Weight | 557.48 |
| CAS Registry Number | 2565657-41-0 |
| SMILES | O=C(N1C[C@@H]([C@H](CC2=CC=CC(Br)=C2)C(N3C(OC[C@@H]3CC4=CC=CC=C4)=O)=O)CC1)OC(C)(C)C |
|
tert-Butyl (R)-3-((S)-1-((S)-4-benzyl-2-oxooxazolidin-3-yl)-3-(3-bromophenyl)-1-oxopropan-2-yl)pyrrolidine-1-carboxylate is a synthetically designed chiral molecule that integrates multiple functional groups and stereocenters. It belongs to a class of compounds commonly used as intermediates in the synthesis of complex organic targets, especially those relevant to medicinal chemistry and stereoselective synthesis. The structure reflects a thoughtful arrangement of protecting groups, chiral centers, and pharmacophores aimed at achieving high levels of control in stereochemical outcomes during synthesis. The molecule contains a pyrrolidine ring substituted at the nitrogen atom with a tert-butoxycarbonyl (Boc) protecting group. The Boc group is widely employed in peptide synthesis and other multistep organic syntheses due to its stability under basic conditions and its ability to be removed under mildly acidic environments. This protective functionality is essential when multiple reactive centers are present, as it allows selective deprotection and further functionalization at later stages. Attached to the 3-position of the pyrrolidine ring is a chiral side chain bearing two additional stereocenters, making the compound highly stereochemically complex. The side chain connects to a central carbon bearing a brominated aromatic ring (3-bromophenyl) and a carbonyl group. The presence of bromine in the aromatic ring not only adds potential for halogen bonding and modulation of electronic properties, but it also provides a point of reactivity for transition-metal-catalyzed cross-coupling reactions such as Suzuki or Heck reactions. These reactions are central to building molecular complexity in medicinal chemistry. One of the defining structural motifs in the molecule is the oxazolidinone ring system, specifically a 4-benzyl-2-oxooxazolidin-3-yl group. Oxazolidinones are five-membered heterocycles containing both nitrogen and oxygen atoms, and they are particularly significant in asymmetric synthesis. The specific substitution pattern and stereochemistry of this oxazolidinone suggest it is derived from a chiral auxiliary, possibly used in the context of Evans’ auxiliary strategies for enantioselective alkylation, aldol, or Michael reactions. The stereocenters in the oxazolidinone ring help impose stereocontrol on adjacent reactions, enabling the precise construction of further chiral centers with defined configuration. The core of the molecule also contains multiple amide and carbonyl functional groups, enhancing its potential for hydrogen bonding and polarity. These features influence both its solubility in polar organic solvents and its reactivity. The proximity of chiral centers and functional groups allows for interactions that can dictate conformational preferences, often critical in determining the course of chemical reactions or biological interactions. This compound is not a natural product but a synthetic intermediate with carefully selected features for use in asymmetric synthesis. It may be involved in the elaboration of complex natural product analogues, pharmaceutical candidates, or in chiral resolution studies. Due to its high degree of functionalization, it can serve as a platform for structural diversification through selective cleavage of the Boc group, modification of the aromatic ring, or transformations involving the oxazolidinone system. Overall, the compound is representative of a class of multifunctional, chiral building blocks designed for precision chemistry. It highlights the modern synthetic strategy of combining protecting groups, chiral auxiliaries, and halogenated aromatic systems to achieve control over reactivity, selectivity, and structural complexity in organic synthesis. |
| Market Analysis Reports |
| List of Reports Available for tert-Butyl (R)-3-((S)-1-((S)-4-benzyl-2-oxooxazolidin-3-yl)-3-(3-bromophenyl)-1-oxopropan-2-yl)pyrrolidine-1-carboxylate |