Online Database of Chemicals from Around the World
| MolScanner | Singapore | Inquire | ||
|---|---|---|---|---|
 |
+86 18621675448 | |||
 |
marketing@molscanner.com | |||
 |
WhatsApp: 9896 7603 | |||
| Chemical manufacturer since 2025 | ||||
| chemBlink standard supplier since 2025 | ||||
| Classification | Pharmaceutical intermediate >> Heterocyclic compound intermediate >> Pyrimidine compound >> Ketones |
|---|---|
| Name | 7-(Chloromethyl)-3-ethyl-1H-1,5-naphthyridin-2-one |
| Molecular Structure | 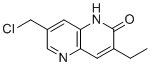 |
| Molecular Formula | C11H11ClN2O |
| Molecular Weight | 222.67 |
| CAS Registry Number | 2589531-78-0 |
| SMILES | CCC1=CC2=C(C=C(C=N2)CCl)NC1=O |
| Density | 1.2±0.1 g/cm3 Calc.* |
|---|---|
| Boiling point | 439.6±45.0 ºC 760 mmHg (Calc.)* |
| Flash point | 219.6±28.7 ºC (Calc.)* |
| Index of refraction | 1.571 (Calc.)* |
| * | Calculated using Advanced Chemistry Development (ACD/Labs) Software. |
| Hazard Symbols |

 GHS05;GHS07 Danger Details
GHS05;GHS07 Danger Details |
|---|---|
| Hazard Statements | H302-H314 Details |
| Precautionary Statements | P264-P270-P271-P280-P303+P361+P353-P304+P340-P305+P351+P338-P310-P330-P331-P363-P403+P233-P501 Details |
| Transport Information | UN 3261 |
| SDS | Available |
|
7-(Chloromethyl)-3-ethyl-1H-1,5-naphthyridin-2-one is a chemical compound belonging to the class of naphthyridines, which are heterocyclic compounds containing a naphthalene ring fused to a pyridine ring. The compound consists of a naphthyridinone backbone with a chloromethyl group at the 7-position and an ethyl group at the 3-position. The discovery and development of naphthyridine derivatives are of significant interest in medicinal chemistry due to their wide range of biological activities. Naphthyridine-based compounds have been explored for their potential as antimicrobial, antiviral, anticancer, and anti-inflammatory agents. The introduction of various substituents, such as the chloromethyl and ethyl groups in this compound, plays a crucial role in modifying the molecule's electronic properties and biological activity. The chloromethyl group at the 7-position is a particularly important feature in this compound. Chloromethyl groups are reactive and can serve as intermediates in further chemical transformations. They are often used in nucleophilic substitution reactions to introduce other functional groups or to attach the molecule to biological targets or surfaces. In medicinal chemistry, such reactive groups can facilitate the design of prodrugs or compounds that release active forms under specific conditions. The ethyl group at the 3-position contributes to the steric and electronic properties of the molecule. Its presence can affect the binding affinity of the compound to its biological targets, such as enzymes or receptors. Additionally, the ethyl group may also improve the lipophilicity of the molecule, potentially enhancing its ability to cross cell membranes and increasing its bioavailability when administered. The naphthyridine scaffold itself is recognized for its versatility in drug design. The 1H-1,5-naphthyridin-2-one structure can be functionalized in various ways to enhance interactions with biological macromolecules. The compound may interact with a variety of enzymes, receptors, or DNA, potentially making it a candidate for use in cancer treatment, antimicrobial therapies, or as an inhibitor of specific enzymes involved in disease processes. Synthesis of 7-(Chloromethyl)-3-ethyl-1H-1,5-naphthyridin-2-one typically involves reactions that modify the naphthyridine core, such as halogenation, alkylation, and nucleophilic substitution. The chloromethyl group is introduced through a chloromethylation reaction, while the ethyl group is typically incorporated by alkylation using an appropriate reagent. The compound's potential applications are broad, especially in drug discovery. Its reactive chloromethyl group makes it a valuable intermediate for further modification, allowing the synthesis of a wide range of derivatives with different biological activities. Additionally, its structure could lend itself to the development of new therapeutic agents aimed at inhibiting specific proteins or processes involved in disease. In summary, 7-(Chloromethyl)-3-ethyl-1H-1,5-naphthyridin-2-one is a promising compound in the field of medicinal chemistry. The combination of the naphthyridine core, chloromethyl group, and ethyl substituent makes it a useful scaffold for the design of bioactive molecules with potential applications in cancer therapy, antimicrobial treatment, and enzyme inhibition. Its reactivity and ability to interact with biological targets offer opportunities for further exploration and development. References 2021. Synthesis of PARP1-DNA Trapper AZD5305. Synfacts, 17(12). DOI: 10.1055/s-0041-1737091 |
| Market Analysis Reports |
| List of Reports Available for 7-(Chloromethyl)-3-ethyl-1H-1,5-naphthyridin-2-one |