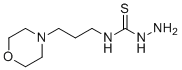
4-(3-Morpholinopropyl)-3-thiosemicarbazide is a specialized organic compound that integrates a morpholine ring and a thiosemicarbazide moiety, rendering it a highly versatile chemical with broad applications in pharmaceutical research, organic synthesis, and materials science. Its synthesis and discovery are tied to ongoing investigations into thiosemicarbazides as multifunctional scaffolds with potential biological and industrial utility.
The compound features a morpholine group, known for its hydrophilic and basic properties, attached via a propyl linker to a thiosemicarbazide core. This unique structure enables 4-(3-Morpholinopropyl)-3-thiosemicarbazide to act as a hydrogen-bond donor and acceptor, contributing to its reactivity and interaction capabilities with various chemical and biological systems. The thiosemicarbazide group, with its sulfur and nitrogen atoms, is especially valuable for chelation, facilitating coordination with metal ions and enabling its use in catalysis and complexation studies.
Pharmaceutical research has identified 4-(3-Morpholinopropyl)-3-thiosemicarbazide as a promising intermediate for the development of bioactive molecules. Its thiosemicarbazide functionality is particularly suited for designing inhibitors of enzymes such as proteases or kinases, where its sulfur and nitrogen atoms can form critical interactions with the active site. Moreover, the morpholine group enhances solubility, making it a favorable choice for medicinal chemistry applications. Derivatives of this compound are being explored for their potential anticancer, antimicrobial, and antiviral activities, reflecting its role as a versatile scaffold in drug discovery.
In organic synthesis, 4-(3-Morpholinopropyl)-3-thiosemicarbazide serves as a key reagent for the preparation of heterocyclic compounds, including thiazoles and triazoles, which are integral to pharmaceutical and agrochemical development. Its reactivity allows it to participate in condensation reactions, cyclizations, and metal-catalyzed transformations, demonstrating its utility as a precursor to complex molecular architectures.
Beyond its pharmaceutical and synthetic applications, the compound holds promise in materials science. Thiosemicarbazides, including this derivative, are being studied as precursors for synthesizing sulfur-rich polymers and coordination complexes with unique electronic and mechanical properties. These materials find applications in fields such as optoelectronics, catalysis, and energy storage.
Research continues to uncover new facets of 4-(3-Morpholinopropyl)-3-thiosemicarbazide, particularly its potential to form supramolecular assemblies and coordinate with transition metals. Its multifunctional nature ensures its relevance across a wide array of scientific disciplines, making it a valuable subject for further exploration.
|





 GHS07 Warning Details
GHS07 Warning Details