Online Database of Chemicals from Around the World
| BOC Sciences | USA | Inquire | ||
|---|---|---|---|---|
 |
+1 (631) 485-4226 | |||
 |
info@bocsci.com | |||
| Chemical manufacturer | ||||
| chemBlink standard supplier since 2010 | ||||
| Glentham Life Sciences | UK | Inquire | ||
|---|---|---|---|---|
 |
+44 (2033) 978-798 | |||
 |
info@glenthamls.com | |||
| Chemical distributor since 2013 | ||||
| chemBlink standard supplier since 2013 | ||||
| Biosynth AG. | Switzerland | Inquire | ||
|---|---|---|---|---|
 |
+41 (71) 858-2020 | |||
 |
welcome@biosynth.ch | |||
| Chemical manufacturer | ||||
| chemBlink standard supplier since 2014 | ||||
| Regent Science Industry Limregent Science Industry Limited | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (755) 8520-1366 | |||
 |
sales@regentsciences.com | |||
| Chemical distributor since 2014 | ||||
| chemBlink standard supplier since 2017 | ||||
| Finetech Industry Limited | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (27) 8746-5837 +86 18971612321 | |||
 |
sales@finetechnology-ind.com | |||
 |
QQ chat | |||
| Chemical manufacturer since 2009 | ||||
| chemBlink standard supplier since 2019 | ||||
| Reymoss (Hebei) Import & Export Trading Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (0319) 365-8752 | |||
 |
chris@reymoss.com | |||
| Chemical distributor since 2021 | ||||
| chemBlink standard supplier since 2021 | ||||
| Shanghai Daji Medical Technology Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 13816245196 | |||
 |
sales1@djpharm.com | |||
| Chemical distributor since 2019 | ||||
| chemBlink standard supplier since 2025 | ||||
| Indofine Chemical Company, Inc. | USA | Inquire | ||
|---|---|---|---|---|
 |
+1 (888) 463-6346 | |||
 |
info@indofinechemical.com | |||
| Chemical manufacturer since 1981 | ||||
| Classification | Biochemical >> Carbohydrate >> Oligosaccharide |
|---|---|
| Name | Amyloheptaose |
| Synonyms | Maltoheptaose |
| Molecular Structure | 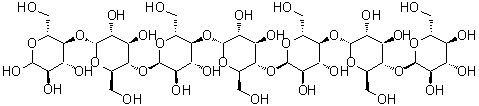 |
| Molecular Formula | C42H72O36 |
| Molecular Weight | 1153.00 |
| CAS Registry Number | 34620-78-5 |
| EC Number | 252-119-4 |
| SMILES | C([C@@H]1[C@H]([C@@H]([C@H]([C@H](O1)O[C@@H]2[C@H](O[C@@H]([C@@H]([C@H]2O)O)O[C@@H]3[C@H](O[C@@H]([C@@H]([C@H]3O)O)O[C@@H]4[C@H](O[C@@H]([C@@H]([C@H]4O)O)O[C@@H]5[C@H](O[C@@H]([C@@H]([C@H]5O)O)O[C@@H]6[C@H](O[C@@H]([C@@H]([C@H]6O)O)O[C@H]([C@@H](CO)O)[C@@H]([C@H](C=O)O)O)CO)CO)CO)CO)CO)O)O)O)O |
| Density | 1.9±0.1 g/cm3 Calc.* |
|---|---|
| Boiling point | 1428.2±65.0 ºC 760 mmHg (Calc.)* |
| Flash point | 817.5±34.3 ºC (Calc.)* |
| Index of refraction | 1.711 (Calc.)* |
| * | Calculated using Advanced Chemistry Development (ACD/Labs) Software. |
| Hazard Symbols |
 GHS07 Warning Details
GHS07 Warning Details |
|---|---|
| Hazard Statements | H302-H315-H319-H335 Details |
| Precautionary Statements | P261-P280-P301+P312-P302+P352-P305+P351+P338 Details |
| SDS | Available |
|
Amyloheptaose is a linear oligosaccharide composed of seven glucose units linked primarily through α-(1→4) glycosidic bonds. Its molecular formula is C42H72O36, with a molecular weight of approximately 1,108.0 g/mol. As a member of the maltooligosaccharide family, amyloheptaose represents an intermediate structure in the enzymatic degradation of starch, positioned between shorter maltodextrins and longer amylose chains. Its linear α-(1→4)-linked glucose sequence imparts flexibility while maintaining a defined chain conformation. The compound is typically prepared through controlled enzymatic hydrolysis of starch using α-amylase, which selectively cleaves α-(1→4) glycosidic bonds to yield oligosaccharides of varying lengths. Fractionation and purification techniques such as size-exclusion chromatography or high-performance liquid chromatography (HPLC) allow isolation of the heptaose fraction. Chemical synthesis of amyloheptaose is also possible via stepwise glycosylation of protected glucose units, though enzymatic methods are generally preferred due to efficiency and stereochemical control. Chemically, amyloheptaose contains multiple hydroxyl groups, which confer high hydrophilicity and enable hydrogen bonding. These hydroxyls are reactive toward derivatization, including acetylation, methylation, and glycosylation, providing opportunities to modify solubility, stability, and interaction properties. The terminal reducing end of amyloheptaose can undergo mutarotation and participate in reactions typical for aldoses, such as Schiff base formation or reduction to sugar alcohols. Amyloheptaose is generally soluble in water due to its hydrophilic character but shows limited solubility in nonpolar organic solvents. Its physical properties, such as viscosity and crystallization behavior, are influenced by chain length and concentration. The oligosaccharide exhibits limited chemical stability under strong acidic or basic conditions, where hydrolysis of glycosidic bonds may occur. Under mild conditions, it is chemically inert and suitable for use in biochemical and synthetic applications. In practical applications, amyloheptaose serves as a model compound in carbohydrate research, enzymology, and glycoscience. It is used to study the specificity and kinetics of amylases, glycosidases, and other carbohydrate-active enzymes. Additionally, amyloheptaose can be a starting material for the synthesis of modified oligosaccharides, conjugates for drug delivery, and glyco-derivatives for biochemical probes. Its defined chain length and stereochemistry make it valuable for investigating the effects of oligosaccharide size on molecular recognition, solubility, and binding interactions. Overall, amyloheptaose is a versatile linear heptasaccharide with multiple reactive hydroxyl groups and a terminal reducing sugar. Its enzymatic origin, defined stereochemistry, and chemical reactivity make it an important intermediate in carbohydrate chemistry, enzymology studies, and the development of functionalized oligosaccharides for research and applied purposes. References 2017. Characterization of the Paenibacillus beijingensis DSM 24997 GtfD and its glucan polymer products representing a new glycoside hydrolase 70 subfamily of 4,6-a-glucanotransferase enzymes. PLOS ONE. DOI: 10.1371/journal.pone.0172622 2015. Glyco-Nanoparticles Made from Self-Assembly of Maltoheptaose-block-Poly(methyl methacrylate): Micelle, Reverse Micelle, and Encapsulation. Biomacromolecules. DOI: 10.1021/acs.biomac.5b00443 2014. Crystal Structure of the Chlamydomonas Starch Debranching Enzyme Isoamylase ISA1 Reveals Insights into the Mechanism of Branch Trimming and Complex Assembly. Journal of Biological Chemistry. DOI: 10.1074/jbc.m114.565044 |
| Market Analysis Reports |
| List of Reports Available for Amyloheptaose |