Online Database of Chemicals from Around the World
| Nanjing Fred Technology Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (25) 8469-6168 | |||
 |
Austin@fredchem.cn | |||
 |
Skype Chat | |||
 |
QQ chat | |||
 |
WeChat: NJFred01 | |||
 |
WhatsApp: +86 17302533743 | |||
| Chemical manufacturer since 2020 | ||||
| chemBlink standard supplier since 2025 | ||||
| Classification | Pharmaceutical intermediate >> Heterocyclic compound intermediate >> Pyridine compound |
|---|---|
| Name | 2-((4-Bromobenzyl)oxy)pyridine |
| Molecular Structure | 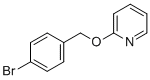 |
| Molecular Formula | C12H10BrNO |
| Molecular Weight | 264.12 |
| CAS Registry Number | 40775-71-1 |
| SMILES | C1=CC=NC(=C1)OCC2=CC=C(C=C2)Br |
| Density | 1.4±0.1 g/cm3 Calc.* |
|---|---|
| Boiling point | 346.7±22.0 ºC 760 mmHg (Calc.)* |
| Flash point | 163.5±22.3 ºC (Calc.)* |
| Index of refraction | 1.605 (Calc.)* |
| * | Calculated using Advanced Chemistry Development (ACD/Labs) Software. |
| Hazard Symbols |
 GHS07 Warning Details
GHS07 Warning Details |
|---|---|
| Hazard Statements | H302-H315-H319-H335 Details |
| Precautionary Statements | P261-P264-P270-P271-P280-P301+P312-P302+P352-P304+P340-P305+P351+P338-P330-P332+P313-P337+P313-P362-P403+P233-P405-P501 Details |
| SDS | Available |
|
2-((4-Bromobenzyl)oxy)pyridine is an organic compound composed of a pyridine ring substituted at the 2-position with a benzyloxy group, where the benzyl moiety bears a bromine atom at the para position. The molecular structure combines the electronic characteristics of both a heteroaromatic pyridine system and an electron-deficient bromobenzyl ether, offering a multifunctional platform for further synthetic elaboration. It is categorized as a heteroaryl ether and is used primarily as an intermediate in organic synthesis and medicinal chemistry. The compound is generally synthesized via a Williamson ether synthesis. This reaction typically involves the deprotonation of 2-hydroxypyridine with a base such as sodium hydride or potassium carbonate, followed by nucleophilic substitution with 4-bromobenzyl halide (usually the bromide or chloride). This approach ensures regioselective O-alkylation at the 2-position of the pyridine ring. The resulting ether bond is robust under a variety of reaction conditions, which makes the molecule suitable for further derivatization. The presence of a bromine substituent at the para position of the benzyl ring provides a reactive site for cross-coupling reactions, such as Suzuki-Miyaura, Buchwald-Hartwig, or Sonogashira couplings. These reactions allow the introduction of a wide range of functional groups or aryl systems at the 4-position of the phenyl ring, thereby expanding the molecular diversity and enabling the development of derivatives with varied biological or physical properties. The pyridine ring itself acts as a coordinating heteroaromatic ligand, and the nitrogen atom at the 1-position can participate in hydrogen bonding or metal coordination. This property is particularly important in the design of ligands for metal complexes or inhibitors of metal-dependent enzymes. The combination of ether and pyridine functionalities makes this compound a versatile scaffold in the design of pharmacophores. In medicinal chemistry, 2-((4-bromobenzyl)oxy)pyridine and its derivatives have been explored for their potential in drug discovery, particularly in areas targeting central nervous system (CNS) disorders and inflammation. The benzyloxy linkage can mimic ether bridges found in natural products and synthetic pharmaceuticals, while the brominated aryl group may influence lipophilicity and binding interactions with hydrophobic regions of biological macromolecules. Additionally, the compound has potential utility in the synthesis of fluorescent probes, agrochemical intermediates, or polymer additives. The rigid and electron-rich pyridine framework can influence the optical or electronic properties of materials when integrated into larger molecular architectures. In this context, the compound may serve as a functional unit in the preparation of more complex molecules for use in materials science. Overall, 2-((4-bromobenzyl)oxy)pyridine is valued for its role as a synthetically accessible intermediate that can undergo selective transformations at both the bromine-substituted aryl ring and the pyridine nitrogen. Its utility lies in the ease with which it can be further derivatized to build structurally diverse and functionally rich compounds across multiple domains of chemical research and development. |
| Market Analysis Reports |
| List of Reports Available for 2-((4-Bromobenzyl)oxy)pyridine |