Online Database of Chemicals from Around the World
| Simagchem Corporation | China | Inquire | ||
|---|---|---|---|---|
 |
+86 13806087780 | |||
 |
sale@simagchem.com | |||
| Chemical manufacturer since 2002 | ||||
| chemBlink standard supplier since 2008 | ||||
| Tianjin Tiandingren Technology Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (22) 5801-5208 | |||
 |
tdr@tiandingren.com | |||
| Chemical distributor since 2010 | ||||
| chemBlink standard supplier since 2013 | ||||
| Hangzhou Leap Chem Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (571) 8771-1850 | |||
 |
market19@leapchem.com | |||
 |
QQ chat | |||
| Chemical manufacturer since 2006 | ||||
| chemBlink standard supplier since 2015 | ||||
| Shandong Qilu Petrochemical Qitai Petrochemical Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 15866261071 | |||
 |
sales@qitaichem.com | |||
| Chemical manufacturer since 2004 | ||||
| chemBlink standard supplier since 2017 | ||||
| Aajiang Jiuzhou Chem Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 13454675544 | |||
 |
jamie@jiuzhou-chem.com | |||
 |
QQ chat | |||
 |
WeChat: +86 18632988332 | |||
 |
WhatsApp: +86 18632988332 | |||
| Chemical manufacturer since 2007 | ||||
| chemBlink standard supplier since 2023 | ||||
| Zibo Feitian International Trading Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (533) 276-3996 | |||
 |
info@feitianindustry.com | |||
| Chemical distributor since 2005 | ||||
| Changshu Jincheng Chemicals Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (512) 5255-9191 5251-2918 | |||
 |
info@jchg.com | |||
| Chemical manufacturer since 2003 | ||||
| Classification | Biochemical >> Common amino acids and protein drugs |
|---|---|
| Name | Sodium sarcosinate |
| Synonyms | N-Methylglycine monosodium salt |
| Molecular Structure | 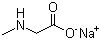 |
| Molecular Formula | C3H6NNaO2 |
| Molecular Weight | 111.07 |
| CAS Registry Number | 4316-73-8 |
| EC Number | 224-338-5 |
| SMILES | CNCC(=O)[O-].[Na+] |
| Density | 1.07 g/cm3 (25 ºC) |
|---|---|
| Solubility | 200�300 g/L (water 25 ºC), slightly soluble (alcohols) |
| Hazard Symbols |
 GHS07 Warning Details
GHS07 Warning Details | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard Statements | H302-H315-H319-H335 Details | ||||||||||||||||
| Precautionary Statements | P261-P264-P270-P271-P280-P301+P312+P330-P302+P352-P304+P340+P312-P305+P351+P338-P332+P313-P337+P313-P362-P403+P233-P405-P501 Details | ||||||||||||||||
| Hazard Classification | |||||||||||||||||
| |||||||||||||||||
| SDS | Available | ||||||||||||||||
|
Sodium sarcosinate, a derivative of sarcosine, is a naturally occurring amino acid that plays an important role in various industrial and biomedical applications. Chemically, it is the sodium salt of N-methylglycine, a compound found in biological tissues as an intermediate in the metabolism of choline and creatine. Its discovery and widespread use have been driven by its amphoteric properties, meaning it can act both as a surfactant and a mild cleansing agent, making it valuable in personal care products and other industries. Sodium sarcosinate was first synthesized in the early 20th century, although sarcosine itself had been known for much longer due to its presence in muscle and brain tissue. The sodium salt form of sarcosine is particularly interesting because of its enhanced solubility and its mild nature, which is suitable for use in products intended for direct contact with the skin and other biological surfaces. One of the most common applications of sodium sarcosinate is in the formulation of personal care products such as shampoos, body washes, and toothpaste. Due to its mildness, it is ideal for sensitive skin and oral care formulations. Its surfactant properties allow it to act as a foaming agent and emulsifier, which helps to create a smooth, consistent texture in these products while also helping to remove oils, dirt, and other impurities from the skin and hair. Unlike harsher surfactants such as sodium lauryl sulfate, sodium sarcosinate is much gentler, reducing the likelihood of irritation or dryness. This has made it a preferred ingredient in "sulfate-free" personal care products, which are increasingly popular among consumers looking for gentler alternatives. In addition to personal care products, sodium sarcosinate has found use in household cleaners and detergents. Its amphoteric nature makes it an effective but non-aggressive cleaning agent, meaning it can remove dirt and stains without damaging surfaces. This has made it particularly valuable in formulations designed for delicate fabrics or surfaces that could be easily scratched or degraded by more aggressive cleaning agents. Beyond consumer products, sodium sarcosinate has applications in biomedical and pharmaceutical research. It is used in laboratory settings as a reagent in biochemical assays, particularly those involving protein or enzyme analysis. Sodium sarcosinate is also sometimes used in drug delivery systems due to its mildness and biocompatibility, ensuring that it does not interfere with the biological activity of the drugs being delivered. Moreover, its role as an intermediate in biochemical pathways makes it a subject of interest in studies related to creatine metabolism, and it is sometimes used in research involving the production of creatine supplements. In recent years, sodium sarcosinate has gained attention in environmental applications. Its biodegradability makes it an environmentally friendly alternative to traditional surfactants that can be persistent in the environment. As concerns about the ecological impact of synthetic chemicals grow, biodegradable compounds like sodium sarcosinate are becoming increasingly important in the formulation of eco-friendly cleaning and personal care products. Sodium sarcosinate continues to be studied for its potential in other industrial applications, including its role in corrosion inhibition and metal treatment processes. Its mild nature combined with its surface-active properties makes it a candidate for use in protecting metals from oxidation and other forms of corrosion without the harsh side effects associated with other chemical inhibitors. References 1971. Sarcosine metabolism in the rat. Biochemical Journal, 124(3). DOI: 10.1042/bj1240587 2018. Poly(sarcosine)-Based Nano-Objects with Multi-Protease Resistance by Aqueous Photoinitiated Polymerization-Induced Self-Assembly (Photo-PISA). Biomacromolecules, 19(11). DOI: 10.1021/acs.biomac.8b01326 |
| Market Analysis Reports |
| List of Reports Available for Sodium sarcosinate |