Online Database of Chemicals from Around the World
| Shanghai Worldyang Chemical Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 13651600618 +86 (21) 5679-5779 | |||
 |
sales7777@worldyachem.com | |||
 |
QQ chat | |||
 |
WeChat: 13651600618 | |||
 |
WhatsApp: +86 13651600618 | |||
| Chemical manufacturer since 2012 | ||||
| chemBlink premium supplier since 2023 | ||||
| Classification | Chemical reagent >> Organic reagent >> Aromatic acid |
|---|---|
| Name | 4-(Trifluoromethyl)benzoic acid |
| Synonyms | alpha,alpha,alpha-Trifluoro-p-toluic acid; 4-Carboxybenzotrifluoride |
| Molecular Structure | 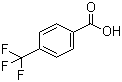 |
| Molecular Formula | C8H5F3O2 |
| Molecular Weight | 190.12 |
| CAS Registry Number | 455-24-3 |
| EC Number | 207-242-8 |
| SMILES | C1=CC(=CC=C1C(=O)O)C(F)(F)F |
| Melting point | 219-222 ºC |
|---|---|
| Boiling point | 247 ºC |
| Flash point | 65.56 ºC |
| Hazard Symbols |
 GHS07 Warning Details
GHS07 Warning Details | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard Statements | H315-H319-H335 Details | ||||||||||||||||||||||||||||
| Precautionary Statements | P261-P264-P264+P265-P271-P280-P302+P352-P304+P340-P305+P351+P338-P319-P321-P332+P317-P337+P317-P362+P364-P403+P233-P405-P501 Details | ||||||||||||||||||||||||||||
| Hazard Classification | |||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||
| SDS | Available | ||||||||||||||||||||||||||||
|
4-(Trifluoromethyl)benzoic acid, a compound of notable chemical significance, was discovered through advanced organic synthesis techniques. By introducing a trifluoromethyl group at the para position of benzoic acid, chemists created a molecule with unique physicochemical properties. This synthesis was part of ongoing efforts to develop new materials and pharmaceuticals, leading to a compound that has since found diverse applications in multiple scientific and industrial fields. 4-(Trifluoromethyl)benzoic acid is widely used in the pharmaceutical industry as a building block for drug synthesis. The trifluoromethyl group enhances the metabolic stability, lipophilicity, and overall pharmacokinetic properties of drug molecules. This makes it an invaluable component in the development of medications for treating a variety of conditions, including cancer, infectious diseases, and neurological disorders. In agriculture, 4-(Trifluoromethyl)benzoic acid is employed in the formulation of herbicides and pesticides. The presence of the trifluoromethyl group increases the efficacy and environmental stability of these agrochemicals, contributing to improved crop protection and yield. This compound serves as a precursor in the production of advanced materials, including polymers and coatings. Fluorinated compounds like 4-(Trifluoromethyl)benzoic acid provide materials with enhanced durability, chemical resistance, and thermal stability, which are essential for applications in electronics, aerospace, and other high-performance industries. Chemists utilize 4-(Trifluoromethyl)benzoic acid in various catalytic processes and organic synthesis reactions. Its unique structural features help in the development of novel catalysts and reagents, facilitating more efficient and selective chemical transformations. This is crucial for the production of complex molecules in pharmaceuticals and other fine chemicals. In analytical chemistry, derivatives of 4-(Trifluoromethyl)benzoic acid are used as internal standards and reference materials. Their well-defined chemical properties allow for precise calibration and validation of analytical methods, enhancing the accuracy and reliability of chemical analyses. The compound is also used in biochemical research to study enzyme interactions and metabolic pathways. Its incorporation into molecular probes and inhibitors helps in elucidating biological mechanisms and discovering new therapeutic targets. References 2022. Aerobic Photooxidation of Toluene Derivatives into Carboxylic Acids with Bromine-Water under Catalyst-Free Conditions. Synlett, 33(15). DOI: 10.1055/a-1887-7885 2019. Photoassisted degradation of trifluoromethyl benzoic acid isomers in aqueous media by Ga2O3 under UVC irradiation. Research on Chemical Intermediates, 45(9). DOI: 10.1007/s11164-019-03852-x 2024. A robust pyrazolate metal-organic framework for integrated perfluorooctanoic acid concentration and degradation. Nature Water, 2(12). DOI: 10.1038/s44221-024-00343-1 |
| Market Analysis Reports |
| List of Reports Available for 4-(Trifluoromethyl)benzoic acid |