Online Database of Chemicals from Around the World
| Shaanxi Scidoor Hi-Tech Biology Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (29) 6296-0300 6296-0309 6296-0305 | |||
 |
sales@scidoor.com | |||
| Chemical manufacturer | ||||
| chemBlink standard supplier since 2006 | ||||
| Xi'an App-Chem Bio (Tech) Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (29) 8831-8908 ex 805 400-6699-003 | |||
 |
sales@appchem.cn | |||
| Chemical manufacturer | ||||
| chemBlink standard supplier since 2007 | ||||
| Shanghai Usea Biotech Company | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (21) 5664-1571 | |||
 |
info@useash.com | |||
| Chemical manufacturer | ||||
| chemBlink standard supplier since 2007 | ||||
| Simagchem Corporation | China | Inquire | ||
|---|---|---|---|---|
 |
+86 13806087780 | |||
 |
sale@simagchem.com | |||
| Chemical manufacturer since 2002 | ||||
| chemBlink standard supplier since 2008 | ||||
| Discovery Fine Chemicals Ltd. | UK | Inquire | ||
|---|---|---|---|---|
 |
+44 (1202) 874-517 | |||
 |
pjc@discofinechem.com | |||
| Chemical manufacturer | ||||
| chemBlink standard supplier since 2009 | ||||
| Extrasynthese Chemical S.A.S. | France | Inquire | ||
|---|---|---|---|---|
 |
+33 (47) 898-2034 | |||
 |
info@extrasynthese.com | |||
| Chemical manufacturer | ||||
| chemBlink standard supplier since 2009 | ||||
| Hangzhou Skyherb Technologies Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (571) 8805-3319 | |||
 |
sales@skyherb.cn | |||
| Chemical manufacturer since 2003 | ||||
| chemBlink standard supplier since 2009 | ||||
| Hefei TNJ Chemical Industry Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (551) 6541-8684 | |||
 |
sales@tnjchem.com | |||
| Chemical manufacturer since 2001 | ||||
| chemBlink standard supplier since 2010 | ||||
| Classification | Biochemical >> Natural biochemical product |
|---|---|
| Name | Apigenin |
| Synonyms | 4',5,7-Trihydroxyflavone; 5,7,4'-Trihydroxyflavone |
| Molecular Structure | 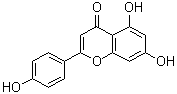 |
| Molecular Formula | C15H10O5 |
| Molecular Weight | 270.24 |
| CAS Registry Number | 520-36-5 |
| EC Number | 208-292-3 |
| SMILES | C1=CC(=CC=C1C2=CC(=O)C3=C(C=C(C=C3O2)O)O)O |
| Solubility | 27 mg/mL (DMSO), 50mg/mL (1M KOH) (Expl.) |
|---|---|
| Density | 1.5±0.1 g/cm3, Calc.* |
| Melting point | 345-350 ºC (Expl.) |
| Index of Refraction | 1.732, Calc.* |
| Boiling Point | 555.5±50.0 ºC (760 mmHg), Calc.* |
| Flash Point | 217.1±23.6 ºC, Calc.* |
| * | Calculated using Advanced Chemistry Development (ACD/Labs) Software. |
| Hazard Symbols |
 GHS07 Warning Details
GHS07 Warning Details | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard Statements | H315-H319-H335 Details | ||||||||||||||||||||||||
| Precautionary Statements | P261-P264-P264+P265-P271-P280-P302+P352-P304+P340-P305+P351+P338-P319-P321-P332+P317-P337+P317-P362+P364-P403+P233-P405-P501 Details | ||||||||||||||||||||||||
| Hazard Classification | |||||||||||||||||||||||||
| |||||||||||||||||||||||||
| SDS | Available | ||||||||||||||||||||||||
|
Apigenin is a naturally occurring flavonoid found in a variety of plants, including parsley, celery, chamomile, and various herbs. It belongs to the class of flavones, characterized by its distinctive yellow color and a chemical structure that includes a benzopyran ring with hydroxyl and methoxy groups. Apigenin has attracted significant attention in the fields of pharmacology, nutrition, and cosmetics due to its wide range of potential health benefits and applications. The discovery of apigenin dates back to the 19th century, when it was first isolated from plants such as parsley. Its presence in numerous plants was later confirmed, and researchers began exploring its biological activities and therapeutic properties. As the interest in natural products for health and wellness grew, apigenin was identified as one of the key components of certain herbal medicines, particularly chamomile, which has long been used as a calming agent. Over time, scientific investigations have provided a deeper understanding of its mechanisms of action, paving the way for its modern-day use in various applications. One of the most significant uses of apigenin is in the realm of herbal medicine. The flavonoid is widely recognized for its calming and anti-anxiety effects. Chamomile tea, which is rich in apigenin, is one of the most popular herbal remedies for promoting relaxation and alleviating insomnia. Apigenin binds to specific receptors in the brain, such as GABA-A receptors, which are involved in inhibiting neuronal activity and promoting relaxation. This interaction contributes to the compound’s sedative effects, making it useful in managing anxiety and sleep disorders. In addition to its calming properties, apigenin has been shown to have anti-inflammatory, antioxidant, and anticancer effects, further enhancing its medicinal value. In the field of cancer research, apigenin has attracted attention for its potential as a chemopreventive agent. Several studies have demonstrated that apigenin can inhibit the growth of cancer cells by interfering with cellular pathways involved in proliferation, apoptosis, and angiogenesis. It has shown activity against various types of cancer, including breast, colon, prostate, and lung cancers. Apigenin’s ability to modulate important signaling pathways, such as the PI3K/Akt/mTOR pathway, makes it a promising candidate for further investigation in cancer therapy. While clinical trials are still ongoing, the compound's anticancer properties make it a potential adjunct to conventional cancer treatments. Furthermore, apigenin is also studied for its neuroprotective properties. Research indicates that it may play a role in protecting the brain from neurodegenerative diseases such as Alzheimer's and Parkinson's disease. Apigenin has been shown to possess antioxidant effects that reduce oxidative stress, which is a key factor in the development of these diseases. Additionally, its ability to reduce inflammation in the brain may contribute to its potential as a therapeutic agent in the prevention and treatment of neurodegenerative disorders. In the cosmetic and skincare industries, apigenin is valued for its antioxidant and anti-inflammatory properties. It is commonly incorporated into skincare formulations due to its ability to protect the skin from free radical damage and reduce the signs of aging. Apigenin helps neutralize the harmful effects of oxidative stress, which can cause premature skin aging, wrinkles, and fine lines. Additionally, its anti-inflammatory effects make it useful in treating conditions such as acne and skin irritation, as it can help reduce redness and swelling. Apigenin is also being explored for its potential in cardiovascular health. Some studies have suggested that apigenin may help reduce blood pressure and improve blood vessel function, contributing to better heart health. Its antioxidant and anti-inflammatory properties may help protect the cardiovascular system from damage caused by oxidative stress and inflammation. Beyond its medical and cosmetic applications, apigenin has also shown potential in agricultural sciences. Due to its bioactivity, it has been considered a natural pesticide and herbicide. Research into its ability to inhibit the growth of certain pests and pathogens suggests that apigenin could serve as a more sustainable and environmentally friendly alternative to synthetic pesticides. The growing interest in apigenin’s therapeutic properties has led to increased research into its mechanisms of action and clinical applications. While many of its potential uses are still being studied, the compound has already shown promise in several areas, from cancer prevention to skin care. As more evidence becomes available, apigenin may become a key player in natural medicine and health-related products. References 2007. Apigenin: A review of its anticancer properties. International Journal of Cancer, 121(10). DOI: 10.1002/ijc.22733 2005. Extraction and analysis of apigenin from plant sources. Journal of Agricultural and Food Chemistry, 53(5). DOI: 10.1021/jf0479867 2008. Synthesis of apigenin derivatives for pharmaceutical applications. Natural Product Research, 22(8). DOI: 10.1080/14786410600898987 |
| Market Analysis Reports |
| List of Reports Available for Apigenin |