Online Database of Chemicals from Around the World
| Zhejiang Arts & Crafts Imp & Exp.co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 571 87238903 | |||
 |
jocelynpan@zjarts.com | |||
| Chemical distributor since 1992 | ||||
| chemBlink standard supplier since 2024 | ||||
| Classification | Organic raw materials >> Heterocyclic compound >> Imidazoles |
|---|---|
| Name | 1-Benzyl-3-methylimidazolium tosylate |
| Molecular Structure | 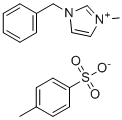 |
| Molecular Formula | C18H20N2O3S |
| Molecular Weight | 344.43 |
| CAS Registry Number | 52461-83-3 |
| SMILES | CC1=CC=C(C=C1)S(=O)(=O)[O-].C[N+]1=CN(C=C1)CC2=CC=CC=C2 |
| Hazard Symbols |
 GHS07 Warning Details
GHS07 Warning Details |
|---|---|
| Hazard Statements | H302+H312-H317-H413 Details |
| Precautionary Statements | P261-P272-P273-P280-P302+P352-P312-P333+P313-P362+P364-P501 Details |
| SDS | Available |
|
1-Benzyl-3-methylimidazolium methanesulfonate is an ionic liquid that belongs to a class of compounds known for their low melting point and unique physicochemical properties. This ionic liquid is composed of a 1-benzyl-3-methylimidazolium cation paired with a methanesulfonate anion. The development and interest in this class of compounds dates back to the late 20th century, when the potential applications of ionic liquids began to be more widely recognized in scientific research and industrial processes. The notable properties of 1-benzyl-3-methylimidazolium methanesulfonate include its thermal stability, low volatility, and ability to dissolve a wide range of organic and inorganic substances. These properties make it an excellent solvent and catalyst for numerous chemical reactions. In addition, it is non-flammable and has good ionic conductivity, which allows it to be used in a variety of advanced applications. In organic synthesis, 1-benzyl-3-methylimidazolium methanesulfonate is frequently used as a catalyst and solvent. It is valuable in the production of fine chemicals and pharmaceuticals due to its ability to promote a variety of reactions such as alkylations, acylations, and cycloadditions. Its catalytic efficiency often results in improved yields and selectivities compared to traditional solvents. This compound is used in electrochemical applications due to its ionic nature and stability. It is particularly beneficial as an electrolyte in electrochemical cells and batteries, helping to improve energy storage and conversion efficiency. 1-Benzyl-3-methylimidazolium methanesulfonate is used in separation and extraction processes due to its excellent solubility capabilities. It is effective in liquid-liquid extractions and can be used to separate complex mixtures and enhance purification processes in chemical manufacturing. This ionic liquid supports the principles of green chemistry, providing an environmentally friendly alternative to traditional organic solvents. Its low volatility reduces emissions of harmful volatile organic compounds (VOCs), thereby minimizing environmental impact and improving the safety of chemical processes. |
| Market Analysis Reports |
| List of Reports Available for 1-Benzyl-3-methylimidazolium tosylate |