Online Database of Chemicals from Around the World
| Shanghai Worldyang Chemical Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 13651600618 +86 (21) 5679-5779 | |||
 |
sales7777@worldyachem.com | |||
 |
QQ chat | |||
 |
WeChat: 13651600618 | |||
 |
WhatsApp: +86 13651600618 | |||
| Chemical manufacturer since 2012 | ||||
| chemBlink premium supplier since 2023 | ||||
| Classification | Chemical reagent >> Organic reagent >> Ester >> Other ester compounds |
|---|---|
| Name | N-Succinimidyl 6-maleimidohexanoate |
| Synonyms | 6-Maleimidohexanoic acid N-hydroxysuccinimide ester |
| Molecular Structure | 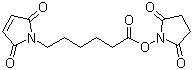 |
| Molecular Formula | C14H16N2O6 |
| Molecular Weight | 308.29 |
| CAS Registry Number | 55750-63-5 |
| EC Number | 637-238-5 |
| SMILES | C1CC(=O)N(C1=O)OC(=O)CCCCCN2C(=O)C=CC2=O |
| Solubility | 10mM (DMSO) |
|---|---|
| Melting point | 71 ºC |
| Hazard Symbols |
 GHS07 Warning Details
GHS07 Warning Details | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard Statements | H315-H319-H335 Details | ||||||||||||||||||||
| Precautionary Statements | P261-P264-P264+P265-P271-P280-P302+P352-P304+P340-P305+P351+P338-P319-P321-P332+P317-P337+P317-P362+P364-P403+P233-P405-P501 Details | ||||||||||||||||||||
| Hazard Classification | |||||||||||||||||||||
| |||||||||||||||||||||
| SDS | Available | ||||||||||||||||||||
|
N-Succinimidyl 6-maleimidocaproate (SMCC) is a widely used compound in bioconjugation and molecular biology. SMCC was originally developed to meet the need for a versatile linker in bioconjugation chemistry. It belongs to the family of N-hydroxysuccinimide (NHS) esters and maleimides, both of which are known for their ability to form stable amide and thioether bonds with primary amines and sulfhydryl groups, respectively. The synthesis of SMCC typically involves coupling maleimide with 6-aminohexanoic acid in the presence of N,N'-dicyclohexylcarbodiimide (DCC) and N-hydroxysuccinimide (NHS). The reaction results in the formation of a maleimide-activated NHS ester, which is then purified and characterized for use in bioconjugation reactions. SMCC has a maleimide group at one end and an NHS ester of a hexanoic acid spacer at the other end. This structure imparts several important properties: The maleimide moiety reacts specifically and efficiently with thiol (-SH) groups under mild conditions to form a stable thioether bond. This reaction is widely used for protein labeling and immobilization. The NHS ester end of SMCC reacts with primary amines (-NH2) on proteins, peptides, or other molecules to form a stable amide bond. This allows SMCC to site-specifically bind to biomolecules. SMCC is primarily used for bioconjugation to attach proteins, peptides, antibodies, or other biomolecules to surfaces, carriers, or to each other. Major applications include: SMCC is used to create antibody-drug conjugates (ADCs), where cytotoxic drugs are conjugated to antibodies via SMCC. This targeted delivery system allows cytotoxic agents to be precisely delivered to cancer cells expressing specific antigens, minimizing off-target effects. Researchers use SMCC to attach fluorescent dyes, biotin, or other markers to proteins or peptides. This helps visualize, purify, or detect biomolecules in biochemical analysis and imaging studies. SMCC modifies surfaces with biomolecules for applications in biosensors, drug delivery systems, and tissue engineering. By conjugating biomolecules to surfaces via SMCC, researchers can control the orientation and density of biomolecules, thereby enhancing functionality and performance. SMCC’s ability to conjugate to both thiol and amine groups makes it suitable for a wide range of bioconjugation applications. Bioconjugation of SMCC typically occurs under physiological conditions (pH 7-8, room temperature), thereby maintaining the activity and stability of the biomolecule. The selective reactivity of SMCC minimizes nonspecific binding and cross-reactivity, ensuring precise bioconjugation. Ongoing research focuses on optimizing SMCC derivatives to enhance their stability, specificity, and biocompatibility for a variety of biomedical applications. Advances in nanotechnology and targeted therapies continue to drive the need for innovative bioconjugation strategies using SMCC and its derivatives. References 2019. Generating the Blood Exposome Database Using a Comprehensive Text Mining and Database Fusion Approach. Environmental Health Perspectives, 127(9). DOI: 10.1289/ehp4713 2024. Hybrid Antimicrobial Coating Based on Conjugate of Hyaluronic Acid with LL-37 Peptide for PEO-Modified Titanium Implants. Russian Journal of Bioorganic Chemistry, 50(2). DOI: 10.1134/s1068162024020225 |
| Market Analysis Reports |
| List of Reports Available for N-Succinimidyl 6-maleimidohexanoate |