Online Database of Chemicals from Around the World
| Shanghai Zhuyao Pharmaceutical Technology Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (021) 5859-6383 | |||
 |
marketing@pharmalego.com | |||
 |
QQ chat | |||
| Chemical manufacturer since 2023 | ||||
| chemBlink standard supplier since 2024 | ||||
| Classification | Chemical reagent >> Organic reagent >> Hydrazine |
|---|---|
| Name | Propane-1-sulfonohydrazide |
| Molecular Structure | 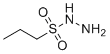 |
| Molecular Formula | C3H10N2O2S |
| Molecular Weight | 138.19 |
| CAS Registry Number | 58032-83-0 |
| EC Number | 873-043-3 |
| SMILES | CCCS(=O)(=O)NN |
| Density | 1.2±0.1 g/cm3, Calc.* |
|---|---|
| Index of Refraction | 1.486, Calc.* |
| Boiling Point | 249.0±23.0 ºC (760 mmHg), Calc.* |
| Flash Point | 104.4±22.6 ºC, Calc.* |
| * | Calculated using Advanced Chemistry Development (ACD/Labs) Software. |
| Hazard Symbols |

 GHS02;GHS07 Danger Details
GHS02;GHS07 Danger Details | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard Statements | H242-H315-H319-H335 Details | ||||||||||||||||||||||||
| Precautionary Statements | P210-P234-P235-P240-P261-P264-P264+P265-P271-P280-P302+P352-P304+P340-P305+P351+P338-P319-P321-P332+P317-P337+P317-P362+P364-P370+P378-P403-P403+P233-P405-P410-P411-P420-P501 Details | ||||||||||||||||||||||||
| Hazard Classification | |||||||||||||||||||||||||
| |||||||||||||||||||||||||
|
Propane-1-sulfonohydrazide is a chemical compound that belongs to the class of sulfonohydrazides, which are widely recognized for their applications in various fields of chemistry, including organic synthesis, medicinal chemistry, and material science. The structure of propane-1-sulfonohydrazide consists of a hydrazide group (-NH-NH2) attached to a propane-1-sulfonyl moiety (-SO2CH2CH3), where the sulfonyl group is responsible for the compound's reactivity. This specific structure provides propane-1-sulfonohydrazide with several important properties that make it useful for diverse chemical processes. The discovery of sulfonohydrazides dates back to the early 20th century when researchers first recognized the potential of hydrazine derivatives for use in organic synthesis. Hydrazine and its derivatives are well-known for their ability to form various types of bonds, making them versatile intermediates in chemical reactions. Propane-1-sulfonohydrazide, as a member of this class, was synthesized with the goal of leveraging the sulfonyl group’s electron-withdrawing properties to influence reactivity in a variety of synthetic applications. One of the primary applications of propane-1-sulfonohydrazide is in organic synthesis, particularly in the formation of hydrazone and azo compounds. The hydrazide group in propane-1-sulfonohydrazide can readily react with carbonyl-containing compounds to form hydrazones, which are valuable intermediates in the synthesis of various organic molecules. Additionally, it can undergo reactions with electrophilic species to form azo compounds, which are used in the production of dyes, pigments, and other organic materials. These reactions are valuable in both industrial and laboratory settings. Propane-1-sulfonohydrazide also plays a role in the synthesis of sulfonylureas, a class of compounds widely used in medicine, particularly for their herbicidal and hypoglycemic properties. Sulfonylureas, which are primarily used to treat Type 2 diabetes, rely on the sulfonyl group for their biological activity. Propane-1-sulfonohydrazide can be used as an intermediate in the preparation of sulfonylurea derivatives, contributing to the development of new and more effective drugs. Another significant application of propane-1-sulfonohydrazide is in the field of material science, where it can serve as a precursor for the synthesis of functional polymers and resins. The hydrazide group can undergo polymerization reactions, leading to the formation of high-molecular-weight materials that may possess unique properties, such as improved strength, flexibility, and chemical resistance. Additionally, the sulfonyl group in propane-1-sulfonohydrazide can provide functionality that enhances the material’s ability to interact with other chemical species, making it useful in coatings, adhesives, and other industrial applications. In the pharmaceutical industry, propane-1-sulfonohydrazide and its derivatives have shown promise as potential agents in cancer therapy. The compound’s ability to interact with certain biomolecules and its reactive nature make it an attractive candidate for drug development, particularly in targeting enzymes or receptors involved in disease pathways. Researchers have investigated its use in combination with other therapeutic agents to enhance anticancer efficacy or to reduce toxicity. Furthermore, propane-1-sulfonohydrazide has been studied for its antimicrobial activity. Like many sulfonyl-based compounds, it exhibits properties that may interfere with bacterial or fungal cell wall synthesis, providing potential as an antimicrobial agent. These applications are still under investigation, but they highlight the broad utility of propane-1-sulfonohydrazide in both medicine and industrial chemistry. In conclusion, propane-1-sulfonohydrazide is a versatile compound with numerous applications in organic synthesis, materials science, and pharmaceuticals. Its ability to act as an intermediate in the formation of hydrazones, azo compounds, and sulfonylureas makes it a valuable tool in both industrial and research settings. Additionally, its potential for use in drug development, particularly in cancer therapy and antimicrobial applications, underscores its importance in advancing chemical and pharmaceutical sciences. |
| Market Analysis Reports |
| List of Reports Available for Propane-1-sulfonohydrazide |