Online Database of Chemicals from Around the World
| Shenyang OllyChem Technology Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (24) 6225-9849 +86 13840042106 | |||
 |
info@ollychem.com oliverdu@ollychem.com | |||
| Chemical manufacturer since 2001 | ||||
| chemBlink premium supplier since 2007 | ||||
| Classification | Organic raw materials >> Carboxylic compounds and derivatives >> Carboxylic esters and their derivatives |
|---|---|
| Name | Methyl 2-(3-(trifluoromethyl)phenoxy)acetate |
| Molecular Structure | 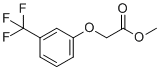 |
| Molecular Formula | C10H9F3O3 |
| Molecular Weight | 234.17 |
| CAS Registry Number | 588-26-1 |
| SMILES | COC(=O)COC1=CC=CC(=C1)C(F)(F)F |
| Hazard Symbols |
 GHS07 Warning Details
GHS07 Warning Details |
|---|---|
| Hazard Statements | H302-H315-H319-H335 Details |
| Precautionary Statements | P261-P280-P301+P312-P302+P352-P305+P351+P338 Details |
| SDS | Available |
|
Methyl 2-(3-(trifluoromethyl)phenoxy)acetate is an important chemical compound notable for its diverse applications in the fields of organic chemistry and pharmaceuticals. This ester is characterized by the presence of a trifluoromethyl group, which imparts unique electronic and steric properties to the molecule. The compound's discovery can be linked to the growing interest in trifluoromethylated compounds, which have gained prominence due to their enhanced biological activities and contributions to drug development. The synthesis of methyl 2-(3-(trifluoromethyl)phenoxy)acetate typically involves the reaction of 3-(trifluoromethyl)phenol with methyl chloroacetate in the presence of a suitable base. This nucleophilic substitution reaction allows for the introduction of the acetate group while preserving the trifluoromethyl moiety. The resulting compound has been subject to extensive characterization through various spectroscopic techniques, including nuclear magnetic resonance (NMR) and mass spectrometry, ensuring its purity and structural integrity. One of the most significant applications of methyl 2-(3-(trifluoromethyl)phenoxy)acetate is in medicinal chemistry, where it serves as a crucial intermediate in the synthesis of bioactive molecules. The trifluoromethyl group is known to enhance the lipophilicity and metabolic stability of compounds, making them more suitable for drug development. As a result, derivatives of this compound have been explored for their potential therapeutic effects, particularly in treating various diseases such as cancer and inflammatory disorders. Additionally, this compound is utilized in agrochemical research, where it may act as a precursor for the development of novel pesticides and herbicides. The unique properties of the trifluoromethyl group often lead to improved efficacy and selectivity in agricultural applications, contributing to the design of more effective and environmentally friendly agricultural chemicals. Moreover, the compound finds its utility in the development of specialty chemicals, including surfactants and polymers. Its ability to modify surface properties makes it a valuable addition to formulations aimed at enhancing performance in various industrial applications. In summary, methyl 2-(3-(trifluoromethyl)phenoxy)acetate represents a versatile compound in synthetic organic chemistry. Its discovery and subsequent applications underscore the importance of trifluoromethylated compounds in enhancing biological activity and utility across multiple fields, from pharmaceuticals to agriculture. |
| Market Analysis Reports |
| List of Reports Available for Methyl 2-(3-(trifluoromethyl)phenoxy)acetate |