Online Database of Chemicals from Around the World
|
CAS: 60-29-7 Product: Diethyl ether No suppilers available. |
| Classification | Organic raw materials >> Ether compounds and their derivatives >> Ether, ether alcohol |
|---|---|
| Name | Diethyl ether |
| Synonyms | Ethyl ether |
| Molecular Structure | 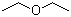 |
| Molecular Formula | C4H10O |
| Molecular Weight | 74.12 |
| CAS Registry Number | 60-29-7 |
| EC Number | 200-467-2 |
| SMILES | CCOCC |
| Density | 0.7±0.1 g/cm3 Calc.*, 0.706 g/mL (Expl.) |
|---|---|
| Melting point | -116 ºC (Expl.) |
| Boiling point | 33.2±3.0 ºC 760 mmHg (Calc.)*, 34.6 ºC (Expl.) |
| Flash point | -40.0 ºC (Calc.)*, -45 ºC (Expl.) |
| Solubility | water: 69 g/L (20 ºC) (Expl.) |
| Index of refraction | 1.361 (Calc.)*, 1.353 (Expl.) |
| * | Calculated using Advanced Chemistry Development (ACD/Labs) Software. |
| Hazard Symbols |

 GHS02;GHS07 DangerGHS02 Details
GHS02;GHS07 DangerGHS02 Details | ||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard Statements | H224-H302-H336 Details | ||||||||||||||||||||||||||||||||||||||||
| Precautionary Statements | P210-P233-P240-P241-P242-P243-P261-P264-P270-P271-P280-P301+P317-P303+P361+P353-P304+P340-P319-P330-P370+P378-P403+P233-P403+P235-P405-P501 Details | ||||||||||||||||||||||||||||||||||||||||
| Hazard Classification | |||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||
| Transport Information | UN 1155 | ||||||||||||||||||||||||||||||||||||||||
| SDS | Available | ||||||||||||||||||||||||||||||||||||||||
|
Diethyl ether, with the chemical formula C2H5OC2H5, is a simple aliphatic ether consisting of two ethyl groups linked by an oxygen atom. It is a volatile, highly flammable liquid with a characteristic sweet odor and low boiling point, which makes it one of the earliest and most widely used organic solvents. The discovery of diethyl ether dates back to the 16th century when it was first prepared by German physician and chemist Valerius Cordus in 1540 through the acid-catalyzed dehydration of ethanol. Its anesthetic properties were recognized in the 19th century, with William T. G. Morton famously demonstrating its use as a surgical anesthetic in 1846. This landmark event established diethyl ether as the first widely used general anesthetic, revolutionizing surgery by enabling pain-free operations. Diethyl ether’s primary applications are as a solvent and an anesthetic. In chemical laboratories and industry, it is valued for its ability to dissolve a wide range of organic compounds due to its moderate polarity and low reactivity. It is commonly used in extractions, recrystallizations, and as a reaction medium for Grignard reagents and other organometallic reactions. Its low boiling point facilitates easy removal by evaporation after completion of a reaction or purification process. Historically, the anesthetic use of diethyl ether had a profound impact on medicine. It allowed surgeons to perform longer and more precise operations without causing extreme pain, significantly reducing surgical mortality and expanding the possibilities of surgical procedures. Although largely replaced by modern anesthetics due to flammability and other side effects, ether remains an important historical milestone in medical practice. Industrial production of diethyl ether involves the acid-catalyzed dehydration of ethanol using sulfuric acid under controlled conditions. The reaction produces ether alongside water, and the product is purified by distillation. Safety is a major consideration because diethyl ether forms highly flammable peroxides upon exposure to air and light, which can be explosive if concentrated. Proper storage and handling are therefore critical. In modern use, diethyl ether continues to serve as a versatile solvent in laboratories, particularly for organic synthesis and purification processes. Its role in the development of anesthesia also secures its place as a historically significant chemical, illustrating the intersection of organic chemistry and medicine. References 1979. De novo fatty acid synthesis and fatty acid elongation catalyzed by subcellular fractions from hog and human aorta. Lipids. DOI: 10.1007/bf02533461 1979. Corticosterone �basal levels� and response to ether anesthesia in rats on a water deprivation regimen. Physiology & Behavior. DOI: 10.1016/0031-9384(79)90225-7 1979. Degradation of aryl phosphates in aquatic environments. Bulletin of Environmental Contamination and Toxicology. DOI: 10.1007/bf02026952 2025. Synthesis, characterization, antimicrobial and antioxidant evaluation of copper(II) complexes with a leucine-derived ligand. Monatshefte f�r Chemie - Chemical Monthly. DOI: 10.1007/s00706-025-03340-6 |
| Market Analysis Reports |
| List of Reports Available for Diethyl ether |