Online Database of Chemicals from Around the World
| Shanghai Worldyang Chemical Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 13651600618 +86 (21) 5679-5779 | |||
 |
sales7777@worldyachem.com | |||
 |
QQ chat | |||
 |
WeChat: 13651600618 | |||
 |
WhatsApp: +86 13651600618 | |||
| Chemical manufacturer since 2012 | ||||
| chemBlink premium supplier since 2023 | ||||
| Classification | Organic raw materials >> Organic fluorine compound >> Fluoropropane series |
|---|---|
| Name | Cyclic AMP |
| Synonyms | 3'-5' Cyclic adenosine monophosphate |
| Molecular Structure | 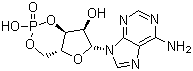 |
| Molecular Formula | C10H12N5O6P |
| Molecular Weight | 329.21 |
| CAS Registry Number | 60-92-4 |
| EC Number | 200-492-9 |
| SMILES | C1[C@@H]2[C@H]([C@H]([C@@H](O2)N3C=NC4=C(N=CN=C43)N)O)OP(=O)(O1)O |
| Melting point | 260 ºC |
|---|---|
| alpha | -53.7 º (c=0.7,water) |
| Water solubility | 50 mg/mL |
| Hazard Symbols |

 HGS05;HGS07 Danger Details
HGS05;HGS07 Danger Details | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard Statements | H314-H315-H318-H319-H335 Details | ||||||||||||||||||||||||
| Precautionary Statements | P260-P261-P264-P264+P265-P271-P280-P301+P330+P331-P302+P352-P302+P361+P354-P304+P340-P305+P351+P338-P305+P354+P338-P316-P317-P319-P321-P332+P317-P337+P317-P362+P364-P363-P403+P233-P405-P501 Details | ||||||||||||||||||||||||
| Hazard Classification | |||||||||||||||||||||||||
| |||||||||||||||||||||||||
| SDS | Available | ||||||||||||||||||||||||
|
Cyclic adenosine monophosphate (cAMP) is a key molecule in cell signaling that was discovered in the mid-20th century and revolutionized our understanding of intracellular communication. The discovery of cAMP by Earl Sutherland in the 1950s marked a major breakthrough, revealing that hormones such as adrenaline trigger biochemical reactions by activating cAMP. cAMP is produced from adenosine triphosphate (ATP) through the action of adenylate cyclase. Upon hormone binding to cell surface receptors, adenylate cyclase is activated, catalyzing the conversion of ATP to cAMP. This cyclic nucleotide then acts as a second messenger, transmitting extracellular signals to intracellular effectors such as protein kinases and ion channels. The versatility of cAMP covers many physiological processes, including metabolism, gene transcription, and neurotransmission. For example, in metabolic pathways, cAMP regulates glycogenolysis and lipolysis induced by glucagon and adrenaline. In nerve cells, it regulates neurotransmitter release and synaptic plasticity, affecting learning and memory. From a pharmacological perspective, cAMP and its analogs have played an important role in developing treatments for a variety of diseases. Forskolin, a natural product that activates adenylate cyclase, is used experimentally to elevate cAMP levels and study its effects on cellular function. In addition, synthetic cAMP analogs such as dibutyryl-cAMP have been used in research to mimic its signaling properties and explore therapeutic potential. Research on cAMP continues to uncover new roles and applications. Its role in diseases such as cancer, cardiovascular disease, and neurological disorders highlights its importance in biomedical research and drug development. By elucidating the mechanisms controlled by cAMP, scientists aim to develop targeted therapies that manipulate its signaling pathways for therapeutic effects. References 1990. The promoter of the human parathyroid hormone gene contains a functional cyclic AMP-response element. Nucleic Acids Research, 18(19). DOI: 10.1093/nar/18.19.5677 1990. CREB regulation of cellular cyclic AMP-responsive and adenovirus early promoters. Journal of Virology, 64(9). DOI: 10.1128/jvi.64.9.4296-4305.1990 2009. Roles of cyclic AMP in regulation of phototaxis in Chlamydomonas reinhardtii. Biologia, 64(6). DOI: 10.2478/s11756-009-0194-4 |
| Market Analysis Reports |
| List of Reports Available for Cyclic AMP |