Online Database of Chemicals from Around the World
| Wuhan Kemi-works Chemical Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (27) 8573-6489 | |||
 |
info@kemiworks.net sales@kemiworks.com | |||
| Chemical manufacturer | ||||
| chemBlink premium supplier since 2011 | ||||
| Classification | Chemical reagent >> Organic reagent >> Ester >> Methyl ester compound |
|---|---|
| Name | Methyl 2-iodobenzoate |
| Molecular Structure | 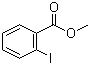 |
| Molecular Formula | C8H7IO2 |
| Molecular Weight | 262.05 |
| CAS Registry Number | 610-97-9 |
| EC Number | 210-243-6 |
| SMILES | COC(=O)C1=CC=CC=C1I |
| Density | 1.8±0.1 g/cm3, Calc.*, 1.73 g/mL (Expl.) |
|---|---|
| Index of Refraction | 1.597, Calc.*, 10604 (Expl.) |
| Boiling Point | 258.8±13.0 ºC (760 mmHg), Calc.*, 149-150 ºC (10mmHg) (Expl.) |
| Flash Point | 110.3±19.8 ºC, Calc.* |
| Hazard Symbols |
 GHS07 Warning Details
GHS07 Warning Details | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard Statements | H315-H319-H335 Details | ||||||||||||||||||||||||
| Precautionary Statements | P261-P264-P264+P265-P271-P280-P302+P352-P304+P340-P305+P351+P338-P319-P321-P332+P317-P337+P317-P362+P364-P403+P233-P405-P501 Details | ||||||||||||||||||||||||
| Hazard Classification | |||||||||||||||||||||||||
| |||||||||||||||||||||||||
| SDS | Available | ||||||||||||||||||||||||
|
Methyl 2-iodobenzoate is an organic compound that belongs to the class of benzoate esters. It consists of a benzoic acid moiety with a methyl group at the ester position and an iodine atom at the second position of the benzene ring. This structure makes it a versatile molecule, often used as a reagent in organic synthesis and in various industrial applications. The discovery of methyl 2-iodobenzoate can be traced to the early studies of halogenated benzoate esters, which are known for their unique reactivity and ability to undergo a variety of substitution reactions. The presence of iodine in the molecule significantly influences its reactivity, making it a valuable compound for synthetic chemistry. One of the primary applications of methyl 2-iodobenzoate is in the field of organic synthesis. The iodine atom in the molecule serves as a leaving group, making it a useful substrate in nucleophilic substitution reactions. This property is particularly advantageous in the preparation of other organic compounds, such as aryl ethers, amides, and other substituted benzoic acid derivatives. Methyl 2-iodobenzoate can be employed in the synthesis of complex molecules, often as an intermediate in the production of pharmaceutical compounds, agrochemicals, and specialty chemicals. Its ability to undergo various substitution reactions, including those with nucleophiles such as alcohols, amines, and thiols, makes it a key building block in synthetic chemistry. In addition to its role in organic synthesis, methyl 2-iodobenzoate is used in the preparation of functionalized materials. For example, it can be incorporated into polymerization reactions to produce materials with specific properties. The presence of iodine in the molecule allows it to participate in radical-based polymerization processes, leading to the formation of functionalized polymers. These polymers can be used in a variety of applications, including coatings, adhesives, and electronic materials. Methyl 2-iodobenzoate’s ability to be incorporated into different types of polymeric structures makes it valuable in the design of materials with tailored properties for use in industrial and technological applications. Another area where methyl 2-iodobenzoate finds application is in the field of medicinal chemistry. Its ability to undergo nucleophilic substitution reactions makes it a useful intermediate for the synthesis of biologically active compounds. For instance, it can be used in the preparation of compounds with antimicrobial, anticancer, and anti-inflammatory properties. The iodine atom in the molecule can also be used to introduce specific functionalities that can enhance the bioactivity and selectivity of the resulting compounds. Researchers have explored the use of methyl 2-iodobenzoate and its derivatives in the development of new therapeutic agents, making it an important compound in the drug discovery process. Furthermore, methyl 2-iodobenzoate has been investigated for its potential use in the development of imaging agents. The iodine atom, being a halogen, has favorable properties for use in radiolabeling applications. By attaching the compound to a targeting moiety, it can be used in imaging techniques such as positron emission tomography (PET) or single-photon emission computed tomography (SPECT). These imaging techniques are essential in medical diagnostics, particularly in oncology, where they are used to track the distribution of drugs or to visualize tumors. The radiolabeling of methyl 2-iodobenzoate opens up potential applications in medical imaging and personalized medicine. In summary, methyl 2-iodobenzoate is a valuable chemical compound with a range of applications in organic synthesis, materials science, medicinal chemistry, and imaging technologies. Its structure, with a methyl ester and iodine substituent, makes it highly reactive and versatile, particularly in nucleophilic substitution reactions. Whether used as an intermediate in the synthesis of pharmaceuticals or as a building block for functionalized materials, methyl 2-iodobenzoate is an important compound in the development of new technologies and therapeutic agents. References 2018. Palladium-Catalyzed Direct Acylation: One-Pot Relay Synthesis of Anthraquinones. Synthesis, 50(20). DOI: 10.1055/s-0037-1610296 2019. Stille Coupling. Science of Synthesis. URL: https://science-of-synthesis.thieme.com/app/text/?id=SD-231-00009 2019. Synthesis of Phenanthridinones by Palladium-Catalyzed Cyclization of N-Aryl-2-aminopyridines with 2-Iodobenzoic Acids in Water. Synlett, 31(01). DOI: 10.1055/s-0039-1691538 |
| Market Analysis Reports |
| List of Reports Available for Methyl 2-iodobenzoate |