Online Database of Chemicals from Around the World
| Eurochim | Russia | Inquire | ||
|---|---|---|---|---|
 |
+7 (812) 320-0093 | |||
 |
danilova@ehim.spb.su | |||
| Chemical manufacturer since 1992 | ||||
| chemBlink standard supplier since 2011 | ||||
| Jiangsu Evergreen New Material Technology Incorporated | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (025) 8491-1160 | |||
 |
flora.wang@cqsgfz.cn | |||
 |
Skype Chat | |||
 |
QQ chat | |||
 |
WeChat: 13605178468 | |||
 |
WhatsApp: +8613605178468 | |||
| Chemical manufacturer since 2010 | ||||
| chemBlink standard supplier since 2024 | ||||
| Scientific Polymer Products, Inc. | USA | Inquire | ||
|---|---|---|---|---|
 |
+1 (585) 265-0413 | |||
 |
custserv@scipoly.com | |||
| Chemical manufacturer | ||||
| Classification | Chemical reagent >> Organic reagent >> Olefins (cyclic and non-cyclic) |
|---|---|
| Name | 4-Methylstyrene |
| Synonyms | 4-Vinyltoluene |
| Molecular Structure | 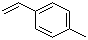 |
| Molecular Formula | C9H10 |
| Molecular Weight | 118.18 |
| CAS Registry Number | 622-97-9 |
| EC Number | 210-762-8 |
| SMILES | CC1=CC=C(C=C1)C=C |
| Density | 0.9±0.1 g/cm3, Calc.*, 0.896 g/mL (Expl.) |
|---|---|
| Melting point | -34 ºC (Expl.) |
| Index of Refraction | 1.551, Calc.*, 1.542 (Expl.) |
| Boiling Point | 172.0±10.0 ºC (760 mmHg), Calc.*, 171-175 ºC (Expl.) |
| Flash Point | 45.6 ºC, Calc.*, 46 ºC (Expl.) |
| * | Calculated using Advanced Chemistry Development (ACD/Labs) Software. |
| Hazard Symbols |



 GHS02;GHS07;GHS08;GHS09 Danger Details
GHS02;GHS07;GHS08;GHS09 Danger Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard Statements | H226-H304-H315-H319-H332-H335-H411-H412 Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Precautionary Statements | P210-P233-P240-P241-P242-P243-P261-P264-P264+P265-P271-P273-P280-P301+P316-P302+P352-P303+P361+P353-P304+P340-P305+P351+P338-P317-P319-P321-P331-P332+P317-P337+P317-P362+P364-P370+P378-P391-P403+P233-P403+P235-P405-P501 Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hazard Classification | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||
| SDS | Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||
|
4-Methylstyrene is an aromatic compound with the chemical formula C9H10, consisting of a benzene ring substituted with a methyl group at the para position relative to the vinyl group. This compound is a member of the styrene family and is primarily used in the production of polymers and resins due to its ability to undergo polymerization. 4-Methylstyrene was first synthesized in the early 20th century and has since become an important intermediate in the chemical industry. It is typically produced by the Friedel–Crafts alkylation of toluene with acetylene or by other similar alkylation reactions. The presence of the methyl group on the benzene ring modifies the properties of the molecule, influencing its reactivity in chemical reactions. The primary use of 4-methylstyrene is in the manufacture of polystyrene and other styrene-based copolymers. It is incorporated into polymer systems to enhance the material's physical properties, such as impact resistance, rigidity, and thermal stability. The polymerization of 4-methylstyrene is commonly carried out through free radical polymerization, where it can be copolymerized with styrene or other monomers to form materials with tailored characteristics. These copolymers are widely used in applications such as automotive parts, electrical components, and household products. In addition to its role in polymer production, 4-methylstyrene is used in the synthesis of resins, adhesives, and coatings. It is also employed as a monomer in the production of high-performance materials that require specific mechanical and chemical properties. The compound's reactivity makes it useful in the production of crosslinked polymers, where the vinyl group can participate in further polymerization or crosslinking reactions. Due to its chemical structure, 4-methylstyrene exhibits certain volatility and is prone to polymerization when exposed to heat or light, which requires careful handling and storage under controlled conditions to prevent unwanted reactions. Stabilizers are often added to prevent premature polymerization during transport or storage. In summary, 4-methylstyrene is a widely used chemical in the polymer and materials industries, valued for its ability to polymerize and copolymerize with other compounds to produce durable and versatile materials. References 2024. M-BTC as efficient catalyst for the synthesis of cyclic organic carbonates assisted tandem by olefin epoxidation and CO2 cycloaddition. Catalysis Letters, 152(11), 2747�2755. DOI: 10.1007/s10562-024-04881-x 2024. Efficient and selective oxidation of alcohols and hydrocarbons catalyzed by oxovanadium(IV) unsymmetrical salophen complex supported on silica-coated CoFe2O4 magnetic nanoparticles. Journal of the Iranian Chemical Society, 21(11), 3157�3166. DOI: 10.1007/s13738-024-03128-1 2024. Covalent attachment of Mn-porphyrin onto functionalized activated carbon for green oxidation of olefins. Journal of the Iranian Chemical Society, 21(10), 2761�2769. DOI: 10.1007/s13738-024-03114-7 |
| Market Analysis Reports |
| List of Reports Available for 4-Methylstyrene |