Online Database of Chemicals from Around the World
| Simagchem Corporation | China | Inquire | ||
|---|---|---|---|---|
 |
+86 13806087780 | |||
 |
sale@simagchem.com | |||
| Chemical manufacturer since 2002 | ||||
| chemBlink standard supplier since 2008 | ||||
| Dishman Pharmaceuticals & Chemicals Limited | USA | Inquire | ||
|---|---|---|---|---|
 |
+1 (732) 560-4300 | |||
 |
bhaveshoza@dishmangroup.com | |||
| Chemical manufacturer | ||||
| chemBlink standard supplier since 2011 | ||||
| Zibo Kunran Enterprises Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (533) 520-0667 | |||
 |
monazhangchem@hotmail.com | |||
 |
QQ chat | |||
| Chemical manufacturer since 2003 | ||||
| chemBlink standard supplier since 2014 | ||||
| Hangzhou Leap Chem Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (571) 8771-1850 | |||
 |
market19@leapchem.com | |||
 |
QQ chat | |||
| Chemical manufacturer since 2006 | ||||
| chemBlink standard supplier since 2015 | ||||
| Neostar United (Changzhou) Industrial Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (519) 8555-7386 +86 18015025600 | |||
 |
marketing1@neostarunited.com | |||
| Chemical distributor since 2014 | ||||
| chemBlink standard supplier since 2020 | ||||
| Guizhou Jianye Yongrong Technology Co., Ltd. (Changzhou Huadong Chemical Research Institute) | China | Inquire | ||
|---|---|---|---|---|
 |
+86 +13851563211 | |||
 |
sales@huadongchem.com | |||
 |
WeChat: +13851563211 | |||
| Chemical manufacturer since 1999 | ||||
| chemBlink standard supplier since 2025 | ||||
| Wako Pure Chemical Industries, Ltd. | Japan | Inquire | ||
|---|---|---|---|---|
 |
+81 (6) 6203-3741 | |||
 |
wkhk.info@fujifilm.com | |||
| Chemical manufacturer since 1922 | ||||
| Zhejiang Xianju Application Chemical Research Institute | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (576) 8768-8839 8760-1639 8768-9177 | |||
 |
lucychen@chemrole.com | |||
| Chemical manufacturer since 1993 | ||||
| Classification | Chemical reagent >> Organic reagent >> Phosphorus halide |
|---|---|
| Name | Propyltriphenylphosphonium bromide |
| Synonyms | n-Propyltriphenylphosphonium bromide |
| Molecular Structure | 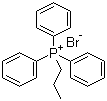 |
| Molecular Formula | C21H22P.Br |
| Molecular Weight | 385.28 |
| CAS Registry Number | 6228-47-3 |
| EC Number | 228-330-2 |
| SMILES | CCC[P+](C1=CC=CC=C1)(C2=CC=CC=C2)C3=CC=CC=C3.[Br-] |
| Melting point | 235-238 ºC (Expl.) |
|---|---|
| Water solubility | soluble |
| Hazard Symbols |
 GHS07 Warning Details
GHS07 Warning Details | ||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard Statements | H315-H319-H335 Details | ||||||||||||||||||||||||||||||||||||||||||||
| Precautionary Statements | P261-P305+P351+P338 Details | ||||||||||||||||||||||||||||||||||||||||||||
| Hazard Classification | |||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||
| SDS | Available | ||||||||||||||||||||||||||||||||||||||||||||
|
Propyltriphenylphosphonium bromide, with the molecular formula C21H21BrP, is a quaternary ammonium salt featuring a triphenylphosphonium cation and a propyl group. This compound is typically synthesized by reacting triphenylphosphine with an alkyl halide such as propyl bromide. The resulting salt appears as a white to light yellow crystalline solid that is soluble in organic solvents such as acetone, methanol, and chloroform. The discovery of propyltriphenylphosphonium bromide is tied to the broader development of phosphonium salts, which have been widely studied due to their utility in organic synthesis. These compounds are particularly useful in the generation of reactive intermediates, such as ylides, which play a crucial role in a number of synthetic transformations. One of the primary applications of propyltriphenylphosphonium bromide is in the Wittig reaction, a method used to synthesize alkenes from carbonyl compounds. In this reaction, the phosphonium salt undergoes deprotonation by a strong base to form a phosphonium ylide. This ylide is a highly reactive species that can then react with a carbonyl compound, such as an aldehyde or ketone, leading to the formation of a carbon-carbon double bond. The Wittig reaction is widely utilized in organic chemistry to prepare alkenes, which are important building blocks in the synthesis of a variety of organic compounds, including pharmaceuticals, agrochemicals, and polymers. Propyltriphenylphosphonium bromide is also used in other reactions that involve phosphonium ylides, such as in the formation of complex organic molecules. The ability of these ylides to participate in nucleophilic substitutions and additions makes them versatile reagents for the synthesis of a wide range of chemical compounds. In addition to its role in organic synthesis, propyltriphenylphosphonium bromide has applications in phase transfer catalysis. Phase transfer catalysis is a technique used to facilitate reactions between two substances that are in different phases, typically where one reactant is in an aqueous phase and the other is in an organic phase. Propyltriphenylphosphonium bromide can assist in the transport of ionic species between these phases, thereby improving reaction rates and efficiency. This makes it useful in processes such as alkylation, esterification, and other transformations involving ionic reactants. The compound is also studied in the context of organophosphorus chemistry, which includes the synthesis of various phosphonium salts and their derivatives. These compounds are valuable for the development of new materials, including phosphonium-based ionic liquids, which are increasingly used in catalysis, electrochemistry, and solvent extraction processes due to their unique properties. As with other phosphonium salts, propyltriphenylphosphonium bromide should be handled with care. It can be irritating to the skin, eyes, and respiratory system, and appropriate safety measures, including the use of gloves, goggles, and working in a well-ventilated area, should be observed. In summary, propyltriphenylphosphonium bromide is an important chemical compound used in organic synthesis, particularly in the Wittig reaction and phase transfer catalysis. Its ability to generate reactive ylides and facilitate the transfer of ions between different phases makes it a valuable reagent in the synthesis of alkenes and other organic compounds. Additionally, it plays a significant role in the development of organophosphorus chemistry and the design of new materials for advanced chemical applications. References 2014 Incorporation of triphenylphosphonium functionality improves the inhibitory properties of phenothiazine derivatives in Mycobacterium tuberculosis Bioorganic & Medicinal Chemistry DOI: 10.1016/j.bmc.2014.07.050 2024 Synthesis of oxanorbornene-based phosphonium polymeric ionic liquids (PILs) and investigation of their electrical properties Mater. Adv. DOI: 10.1039/D3MA00630A 2018 Dehydrochlorination of 1,2-dichloroethane over a tetraphenylphosphonium chloride-supported carbon catalyst New J. Chem. DOI: 10.1039/C8NJ02580K |
| Market Analysis Reports |
| List of Reports Available for Propyltriphenylphosphonium bromide |