Online Database of Chemicals from Around the World
| Hangzhou Ich Biofarm Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (571) 2818-6870 | |||
 |
specialchem1@ichemie.com | |||
 |
QQ chat | |||
| Chemical manufacturer since 2006 | ||||
| chemBlink standard supplier since 2011 | ||||
| Classification | Chemical reagent >> Organic reagent >> Sulfonate / sulfinate |
|---|---|
| Name | 3-(2,3,3-Trimethyl-5-sulfo-3H-indol-1-ium-1-yl)propane-1-sulfonate |
| Synonyms | 2,3,3-Trimethyl-1-(3-sulfopropyl)-3H-indolium-5-sulfonate |
| Molecular Structure | 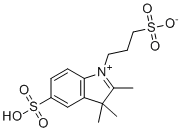 |
| Molecular Formula | C14H19NO6S2 |
| Molecular Weight | 361.43 |
| CAS Registry Number | 76588-81-3 |
| SMILES | CC1=[N+](C2=C(C1(C)C)C=C(C=C2)S(=O)(=O)O)CCCS(=O)(=O)[O-] |
|
3-(2,3,3-Trimethyl-5-sulfo-3H-indol-1-ium-1-yl)propane-1-sulfonate is a zwitterionic organic compound featuring both a positively charged quaternary indolium core and two sulfonate groups, each contributing a negatively charged sulfonic acid moiety. The molecular formula is C14H20N1O6S2. This compound belongs to the class of sulfoindolium derivatives and is commonly used in dye chemistry and as a building block in the synthesis of fluorescent or chromogenic agents. The structure contains a substituted indole ring where the nitrogen is quaternized to form an indolium ion. The 2,3,3-trimethyl substitution pattern on the ring confers both steric hindrance and increased hydrophobic character, while the sulfonic acid group at the 5-position of the indole ring and another sulfonate group on the terminal carbon of the 3-carbon side chain significantly enhance water solubility. The result is a highly polar molecule that maintains stable ionic character under a wide range of pH conditions. This compound is typically synthesized through the sulfonation of indole or indoline derivatives, followed by N-alkylation and the introduction of a sulfonated side chain. The overall design ensures the molecule exists predominantly in a zwitterionic state, where the positive charge on the nitrogen is counterbalanced by the negative charges on the sulfonate groups. Applications of 3-(2,3,3-trimethyl-5-sulfo-3H-indol-1-ium-1-yl)propane-1-sulfonate are largely found in the field of dye and probe development. The indolium core is known for its conjugated π-system, which can be extended through condensation with other aromatic aldehydes or chromophores to yield polymethine dyes, including cyanines and hemicyanines. These dyes are widely used in fluorescence imaging, biological labeling, photodynamic therapy, and optical materials. The sulfonate groups improve aqueous solubility and reduce non-specific binding in biological environments. The compound also serves as a key intermediate in the synthesis of asymmetrical cyanine dyes. When combined with heterocycles like benzothiazoles or quinolinium groups, it contributes to the synthesis of fluorescent probes that are highly responsive to changes in local polarity, pH, or biomolecular interactions. Additionally, the sulfonic acid functionalities can be exploited for further derivatization or for covalent conjugation to biomolecules, nanoparticles, or polymers. Physically, this compound typically appears as a solid with good thermal stability. It is highly soluble in water and exhibits strong absorption in the visible to near-infrared region when part of an extended dye system. Due to its ionic nature, it is non-volatile and generally non-flammable. In terms of safety and handling, the sulfonate groups make it relatively nontoxic and non-irritant, although appropriate laboratory practices should always be observed. Its ionic properties also make it non-bioaccumulative and biodegradable under standard environmental conditions. In summary, 3-(2,3,3-trimethyl-5-sulfo-3H-indol-1-ium-1-yl)propane-1-sulfonate is a zwitterionic indolium-based compound used extensively in the synthesis of water-soluble dyes and fluorescent probes. Its structural features make it a versatile and important building block for applications in bioimaging, diagnostics, and materials science. References 2011. By Alkylation. Science of Synthesis. URL: https://science-of-synthesis.thieme.com/app/text/?id=SD-127-00100 |
| Market Analysis Reports |
| List of Reports Available for 3-(2,3,3-Trimethyl-5-sulfo-3H-indol-1-ium-1-yl)propane-1-sulfonate |