Ammonium persulfate is an inorganic salt composed of ammonium cations and persulfate anions, with the chemical formula (NH4)2S2O8. It was first identified in the 19th century as part of the broader study of persulfate compounds, which are known for their strong oxidizing properties. The commercial production of ammonium persulfate is generally achieved by the electrolytic oxidation of concentrated ammonium sulfate solutions in sulfuric acid. This method involves passing an electric current through the solution, leading to the formation of the persulfate ion (S2O82−) at the anode, which subsequently pairs with ammonium ions to form ammonium persulfate crystals. The salt appears as a white, odorless, crystalline solid that is highly soluble in water and stable under normal conditions but decomposes upon heating to release free radicals.
The strong oxidizing nature of ammonium persulfate has made it a valuable reagent in both industrial and laboratory settings. One of its most significant applications is as a radical initiator in polymer chemistry. When heated or dissolved in water, ammonium persulfate decomposes to generate sulfate radicals (SO4•−), which can initiate free radical polymerization. This property is widely exploited in the synthesis of polymers such as polyacrylamide and polystyrene. The controlled initiation of radical polymerization by ammonium persulfate allows for the production of polymers with desirable molecular weights and structural features, essential for applications ranging from water treatment flocculants to gel electrophoresis media.
In addition to its role in polymer synthesis, ammonium persulfate serves as an oxidizing agent in organic synthesis. It facilitates a variety of reactions, including oxidative coupling, desulfurization, and selective oxidation processes. These applications leverage its ability to generate radicals under mild conditions, enabling the modification of organic molecules in both academic research and industrial processes.
Ammonium persulfate is also employed in the electronics industry, particularly in the manufacture of printed circuit boards. Its oxidizing properties assist in etching copper surfaces by dissolving unwanted copper layers, providing precision and efficiency in the fabrication process. Furthermore, in environmental chemistry, ammonium persulfate has been used in advanced oxidation processes for water and wastewater treatment. The sulfate radicals generated from its activation are effective in degrading organic pollutants, making it a useful reagent for contaminant removal and water purification technologies.
The versatility of ammonium persulfate arises from its reliable radical generation and strong oxidation potential, combined with relatively straightforward handling and solubility. Its applications continue to expand with ongoing research into radical chemistry and sustainable industrial processes. The safety profile of ammonium persulfate requires careful handling, as it is a strong oxidizer and can cause irritation upon contact with skin or respiratory systems. However, with appropriate precautions, it remains a cornerstone chemical in polymerization, synthesis, and environmental remediation.
References
1984. Shifts of delayed immune response to persulphates and other allergens. Contact Dermatitis, 11(2).
DOI: 10.1111/j.1600-0536.1984.tb00963.x
1971. [Localization of the IgM-fraction in discontinuous polyacrylamide electrophoresis columns]. Blut, 23(2).
DOI: 10.1007/bf01632737
1971. Comparative studies of fructose 1,6-diphosphate aldolase from Escherichia coli 518 and Lactobacillus casei var. rhamnosus ATCC 7469. Antonie van Leeuwenhoek, 37(1).
DOI: 10.1007/bf02218464
|

















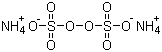


 GHS03;GHS07;GHS08 Danger Details
GHS03;GHS07;GHS08 Danger Details