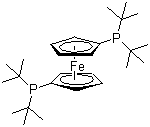
1,1'-Bis(di-tert-butylphosphino)ferrocene, often abbreviated as dtbpf, is a highly significant compound in organometallic chemistry due to its role as a versatile ligand. This chemical substance consists of a ferrocene core with two di-tert-butylphosphino groups attached to the cyclopentadienyl rings. The ferrocene structure imparts both stability and unique electronic properties to the ligand, making it valuable for various catalytic applications.
The discovery of dtbpf emerged from the need for ligands that offer strong stabilization and electronic tuning in transition metal catalysis. The di-tert-butylphosphino groups provide substantial steric protection around the metal center, which helps to prevent catalyst deactivation and enhance selectivity. This steric bulk is crucial for reactions involving bulky substrates or under demanding reaction conditions.
1,1'-Bis(di-tert-butylphosphino)ferrocene is predominantly used in palladium-catalyzed cross-coupling reactions, such as the Suzuki-Miyaura and Heck reactions. These reactions are foundational in organic synthesis, enabling the formation of carbon-carbon bonds necessary for constructing complex organic molecules. The presence of dtbpf in these reactions improves catalyst stability and performance, leading to higher yields and better control over reaction outcomes.
Additionally, dtbpf is utilized in other catalytic processes, including hydrogenation and olefin polymerization. Its ability to stabilize transition metal centers and modulate electronic properties makes it an excellent choice for optimizing various catalytic systems. The compound's versatility extends to its use in asymmetric synthesis, where it aids in the creation of enantioselective products.
The development of 1,1'-Bis(di-tert-butylphosphino)ferrocene highlights the advancements in ligand design aimed at enhancing catalytic efficiency. Its unique combination of steric and electronic effects makes it a valuable tool in both research and industrial applications. By offering improved stability and selectivity, dtbpf plays a crucial role in advancing the field of organometallic chemistry.
Overall, 1,1'-Bis(di-tert-butylphosphino)ferrocene exemplifies the impact of strategic ligand design on catalytic performance, demonstrating its importance in modern chemical synthesis.
References
2013. Rh-Catalyzed Aldehyde-Aldehyde Cross-Aldol Reaction under Base-Free Conditions: In Situ Aldehyde-Derived Enolate Formation through Orthogonal Activation. Chemistry - An Asian Journal, 8(12).
DOI: 10.1002/asia.201300928
|























 GHS07 Warning Details
GHS07 Warning Details