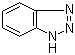
1H-Benzotriazole is a chemical compound that belongs to the class of heterocyclic compounds, featuring a fused benzene and triazole ring structure. It was first synthesized in the early 20th century as researchers sought to explore the properties and potential applications of triazole derivatives. The initial discovery of benzotriazole is often attributed to its relevance in the field of coordination chemistry, particularly due to its ability to form stable complexes with various metal ions.
The synthesis of 1H-benzotriazole typically involves the cyclization of ortho-aminobenzoic acid or its derivatives with hydrazine or hydrazine derivatives under acidic conditions. This process yields a compound characterized by its distinctive triazole moiety, which imparts unique chemical properties. The compound exists in two tautomeric forms, but the 1H-benzotriazole form is the more stable and commonly encountered structure in applications.
One of the primary applications of 1H-benzotriazole is in the field of corrosion inhibition. It is widely used as a corrosion inhibitor for metals and alloys, particularly in the protection of copper and its alloys. The compound forms a protective film on metal surfaces, preventing oxidation and deterioration caused by environmental factors. This application is critical in various industries, including automotive, aerospace, and marine, where metal components are exposed to corrosive environments.
In addition to its role in corrosion inhibition, 1H-benzotriazole has found applications in the field of organic synthesis as a building block for various chemical compounds. It serves as an effective reagent in the synthesis of pharmaceuticals, agrochemicals, and dyes. Its ability to participate in various chemical reactions, such as nucleophilic substitutions and cycloadditions, makes it a valuable intermediate in the production of complex organic molecules.
Moreover, 1H-benzotriazole is utilized in the field of photoprotection. It has been shown to absorb ultraviolet (UV) radiation, making it a useful additive in sunscreens and other cosmetic formulations. By absorbing harmful UV rays, it helps protect the skin from damage caused by sun exposure, thus contributing to the effectiveness of personal care products.
Safety considerations are important when handling 1H-benzotriazole. While it is generally considered to have low toxicity, appropriate safety measures should be taken during its handling and use, as exposure may lead to irritation of the skin and eyes. It is essential to follow safety guidelines and use personal protective equipment when working with this compound.
In summary, 1H-benzotriazole is a versatile compound with diverse applications in corrosion inhibition, organic synthesis, and photoprotection. Its discovery and subsequent development have led to significant contributions in various industrial sectors, showcasing its importance as a valuable chemical substance.
|



















 GHS07;GHS09 Warning Details
GHS07;GHS09 Warning Details