Online Database of Chemicals from Around the World
| Simagchem Corporation | China | Inquire | ||
|---|---|---|---|---|
 |
+86 13806087780 | |||
 |
sale@simagchem.com | |||
| Chemical manufacturer since 2002 | ||||
| chemBlink standard supplier since 2008 | ||||
| Tianjin Zhongxin Chem-tech Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (22) 6688-0623 | |||
 |
sales@tjzxchem.com | |||
| Chemical manufacturer since 2007 | ||||
| chemBlink standard supplier since 2009 | ||||
| Hefei TNJ Chemical Industry Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (551) 6541-8684 | |||
 |
sales@tnjchem.com | |||
| Chemical manufacturer since 2001 | ||||
| chemBlink standard supplier since 2010 | ||||
| BOC Sciences | USA | Inquire | ||
|---|---|---|---|---|
 |
+1 (631) 485-4226 | |||
 |
info@bocsci.com | |||
| Chemical manufacturer | ||||
| chemBlink standard supplier since 2010 | ||||
| Eastar Chemical Corporation | USA | Inquire | ||
|---|---|---|---|---|
 |
+1 800-898-2436 | |||
 |
info@eastarchem.com | |||
| Chemical manufacturer since 1989 | ||||
| chemBlink standard supplier since 2014 | ||||
| Hangzhou Leap Chem Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (571) 8771-1850 | |||
 |
market19@leapchem.com | |||
 |
QQ chat | |||
| Chemical manufacturer since 2006 | ||||
| chemBlink standard supplier since 2015 | ||||
| Shanghai Future Industrial Limited By Share Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 400-0066-400 | |||
 |
sales@jonln.com | |||
| Chemical distributor since 2011 | ||||
| chemBlink standard supplier since 2015 | ||||
| Anhui Royal Chemical Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (25) 8663-9253 | |||
 |
zoey.zhao@royal-chem.com | |||
| Chemical manufacturer since 2008 | ||||
| chemBlink standard supplier since 2019 | ||||
| Classification | Organic raw materials >> Amino compound >> Cycloalkylamines, aromatic monoamines, aromatic polyamines and derivatives and salts |
|---|---|
| Name | N,N-Dimethyl-4-methylaniline |
| Synonyms | N,N-Dimethyl-p-toluidine; 4-Dimethylaminotoluene |
| Molecular Structure | 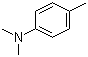 |
| Molecular Formula | C9H13N |
| Molecular Weight | 135.21 |
| CAS Registry Number | 99-97-8 |
| EC Number | 202-805-4 |
| SMILES | CC1=CC=C(C=C1)N(C)C |
| Density | 0.937 g.mL (4 ºC) |
|---|---|
| Melting point | -25 ºC |
| Boiling point | 211 ºC |
| Refractive index | 1.547 |
| Flash point | 83 ºC |
| Hazard Symbols |


 GHS06;GHS08;GHS09 Danger Details
GHS06;GHS08;GHS09 Danger Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard Statements | H301+H311+H331-H301-H311-H331-H373-H410-H412 Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Precautionary Statements | P260-P261-P262-P264-P270-P271-P273-P280-P301+P316-P302+P352-P304+P340-P316-P319-P321-P330-P361+P364-P391-P403+P233-P405-P501 Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hazard Classification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SDS | Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
N,N-Dimethyl-4-methylaniline, also known as 4-methyl-N,N-dimethylaniline, is an organic compound classified as a tertiary amine and aromatic amine. It features a methyl group at the para position of the aniline ring, along with two dimethylamino groups attached to the nitrogen atom. The compound has garnered attention since its discovery in the mid-20th century, primarily due to its applications in various industrial processes and as an important intermediate in chemical synthesis. The synthesis of N,N-dimethyl-4-methylaniline typically involves the methylation of 4-methylaniline using dimethyl sulfate or other methylating agents in the presence of a suitable base. This reaction allows for the introduction of the two methyl groups, resulting in a compound that retains the reactivity of the aniline moiety while benefiting from the electron-donating properties of the dimethylamino groups. The product is usually obtained as a clear liquid with a characteristic amine odor, soluble in organic solvents. N,N-Dimethyl-4-methylaniline is predominantly utilized as a dye intermediate, particularly in the production of azo dyes and other coloring agents. Its ability to undergo electrophilic substitution reactions makes it a valuable building block for synthesizing various azo compounds, which are widely used in textiles, inks, and plastics. The dye industry has benefited significantly from this compound, as it provides vivid colors and stability in final products. Beyond its role in dye production, N,N-dimethyl-4-methylaniline is employed in the formulation of certain types of resins and plastics. The compound can participate in polymerization reactions, enhancing the mechanical properties and thermal stability of the resulting materials. As a result, it is utilized in the manufacturing of coatings, adhesives, and composite materials, contributing to improved performance and durability. In addition to industrial applications, N,N-dimethyl-4-methylaniline is also of interest in the field of pharmaceuticals. It can serve as a precursor in the synthesis of various pharmaceutical compounds, leveraging its reactive functional groups to create new medicinal agents. Researchers continue to explore its potential in developing novel drugs and therapeutic agents. Despite its versatility, it is important to consider safety and environmental aspects when handling N,N-dimethyl-4-methylaniline. The compound is classified as harmful if ingested or inhaled and can cause skin irritation. Proper safety precautions, such as the use of personal protective equipment and adequate ventilation, are essential when working with this chemical in industrial settings. Regulatory agencies have established guidelines to ensure the safe use of N,N-dimethyl-4-methylaniline in various applications. The compound has been subject to ongoing research to evaluate its potential health effects, leading to increased awareness and safety measures in workplaces where it is used. In summary, N,N-dimethyl-4-methylaniline is an important chemical compound with diverse applications, particularly in the dye, resin, and pharmaceutical industries. Its ability to act as an intermediate in chemical synthesis has made it a valuable asset in various formulations. Continued research and awareness regarding safety and environmental impacts will ensure its responsible use in industrial and pharmaceutical applications. References 2024. Highly Effective Zirconia/γ-Alumina for Continuous Vapor Phase N-methylation of Aniline. Catalysis Letters, 154(7). DOI: 10.1007/s10562-023-04568-9 2023. Photoinitiating systems based on 1-hexadecylisatin derivatives. Russian Chemical Bulletin, 72(8). DOI: 10.1007/s11172-023-3955-2 2022. Organic Nitrating Reagents. Synthesis, 54(4). DOI: 10.1055/s-0040-1719905 |
| Market Analysis Reports |
| List of Reports Available for N,N-Dimethyl-4-methylaniline |