(S)-3-Aminobutanenitrile hydrochloride is a chiral organic compound with significant relevance in synthetic chemistry and pharmaceutical development. It is widely utilized as a versatile building block in the synthesis of biologically active molecules and intermediates due to its functional groups and stereochemical properties.
The discovery of (S)-3-aminobutanenitrile hydrochloride stemmed from the growing demand for enantiomerically pure compounds in pharmaceutical and agrochemical industries. Its synthesis typically involves enantioselective approaches, such as asymmetric hydrogenation or enzymatic resolution of racemic mixtures. Advances in catalysis, particularly with chiral ligands, have enabled efficient and scalable production of the (S)-enantiomer with high optical purity.
This compound’s chemical structure features an amine and nitrile group, which confer significant reactivity and make it an attractive intermediate in organic synthesis. It is frequently employed in the preparation of amino acid derivatives, peptides, and heterocyclic compounds. Its ability to undergo transformations such as hydrolysis, reduction, or condensation allows it to serve as a precursor for a wide range of molecules with pharmaceutical relevance.
In medicinal chemistry, (S)-3-aminobutanenitrile hydrochloride has been studied as a key intermediate in the synthesis of active pharmaceutical ingredients (APIs). Its chiral nature makes it particularly useful in the development of drugs where stereochemistry plays a critical role in biological activity and selectivity. For instance, it is a precursor in the synthesis of certain β-amino acids, which are integral to the design of enzyme inhibitors and receptor agonists.
Beyond pharmaceuticals, this compound finds applications in material sciences and agrochemicals. It can be used to synthesize functionalized polymers or as a starting material for chiral catalysts. In the agrochemical industry, its derivatives are explored for their potential as bioactive agents in pest control or crop enhancement.
Ongoing research on (S)-3-aminobutanenitrile hydrochloride focuses on improving synthetic methodologies to enhance yield, reduce environmental impact, and expand its application scope. Studies also explore its use in green chemistry frameworks, such as catalytic processes involving sustainable solvents or recyclable reagents.
|






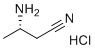
 GHS07 Warning Details
GHS07 Warning Details