Online Database of Chemicals from Around the World
| Shanghai Worldyang Chemical Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 13651600618 +86 (21) 5679-5779 | |||
 |
sales7777@worldyachem.com | |||
 |
QQ chat | |||
 |
WeChat: 13651600618 | |||
 |
WhatsApp: +86 13651600618 | |||
| Chemical manufacturer since 2012 | ||||
| chemBlink premium supplier since 2023 | ||||
| Classification | Organic raw materials >> Organometallic compound >> Organic hafnium, mercury, silver, platinum, etc. |
|---|---|
| Name | Dichloro(1,5-cyclooctadiene)platinum(II) |
| Synonyms | (1,5-Cyclooctadiene)platinum(II) dichloride; Platinum(COD)dichloride |
| Molecular Structure | 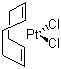 |
| Molecular Formula | C8H12Cl2Pt |
| Molecular Weight | 374.16 |
| CAS Registry Number | 12080-32-9 |
| EC Number | 235-144-5 |
| SMILES | C1/C=C\CC/C=C\C1.Cl[Pt]Cl |
| Melting point | 285 ºC (Decomposes) (Expl.) |
|---|---|
| Water solubility | insoluble |
| Hazard Symbols |
 GHS07 Warning Details
GHS07 Warning Details | ||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard Statements | H315-H319-H335 Details | ||||||||||||||||||||||||||||||||||||||||||||||||
| Precautionary Statements | P261-P264-P264+P265-P271-P280-P302+P352-P304+P340-P305+P351+P338-P319-P321-P332+P317-P337+P317-P362+P364-P403+P233-P405-P501 Details | ||||||||||||||||||||||||||||||||||||||||||||||||
| Hazard Classification | |||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||
| SDS | Available | ||||||||||||||||||||||||||||||||||||||||||||||||
|
Dichloro(1,5-cyclooctadiene)platinum(II) is a coordination complex with the formula PtCl2(COD), where COD stands for 1,5-cyclooctadiene (C8H12). It consists of a platinum(II) center coordinated by two chloride ligands and a bidentate 1,5-cyclooctadiene ligand, which binds through its two alkene groups. This compound appears as a yellow to orange crystalline solid and is soluble in common organic solvents such as dichloromethane and chloroform. The compound was first synthesized during studies of platinum organometallic chemistry in the mid-20th century, when researchers explored complexes of platinum with unsaturated hydrocarbons. The use of cyclooctadiene as a ligand helped stabilize the platinum(II) center, enabling the isolation and characterization of a variety of platinum complexes. Structural determination by X-ray crystallography showed that the platinum center adopts a square planar geometry typical of d8 metal ions, with the COD ligand coordinating in a chelating fashion and the two chloride ligands completing the coordination sphere. Dichloro(1,5-cyclooctadiene)platinum(II) is commonly prepared by reacting potassium tetrachloroplatinate(II) (K2PtCl4) with 1,5-cyclooctadiene under reflux conditions in an aqueous or organic medium. The reaction proceeds through ligand substitution, where two chloride ions are retained while the COD ligand binds to platinum, resulting in the discrete complex. This complex is widely used as a versatile precursor in platinum-based organometallic synthesis and catalysis. Its relatively labile COD ligand can be displaced by other ligands such as phosphines, amines, or olefins, facilitating the preparation of a broad range of platinum complexes with tailored properties. Such complexes find applications in homogeneous catalysis, including hydrosilylation, hydrogenation, and carbon-carbon bond-forming reactions. In addition, dichloro(1,5-cyclooctadiene)platinum(II) serves as a starting material for the synthesis of platinum-containing polymers, materials for electronics, and medicinal compounds. Its well-defined structure and solubility make it suitable for detailed mechanistic studies in organometallic chemistry. Handling of this platinum complex requires caution due to the toxicity of platinum compounds and their potential environmental impact. It may cause skin and respiratory irritation, and prolonged exposure should be avoided. Proper personal protective equipment and ventilation are recommended during its use. In summary, dichloro(1,5-cyclooctadiene)platinum(II) is a stable, square planar platinum(II) complex widely utilized as a synthetic precursor in organometallic chemistry and catalysis. Its coordination with the COD ligand enables easy ligand substitution, supporting diverse applications in synthesis and material science. References 2024. Synthesis of a Novel Platinum Catalyst and Its Application in the Photoactivated Hydrosilylation Reaction. Silicon, 16(15). DOI: 10.1007/s12633-024-03103-8 2020. Platinum Atoms Dispersed in Single-chain Polymer Nanoparticles. Chinese Journal of Polymer Science, 39(2). DOI: 10.1007/s10118-021-2499-x 2011. A kinetic investigation into the rate of chloride substitution from chloro terpyridine platinum(II) and analogous complexes by a series of azole nucleophiles. Transition Metal Chemistry, 36(5). DOI: 10.1007/s11243-011-9507-x |
| Market Analysis Reports |
| List of Reports Available for Dichloro(1,5-cyclooctadiene)platinum(II) |