Online Database of Chemicals from Around the World
| Capot Chemical Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (571) 8558-6718 +86 13336195806 | |||
 |
capotchem@gmail.com sales@capotchem.com | |||
 |
QQ chat | |||
| Chemical manufacturer | ||||
| chemBlink standard supplier since 2006 | ||||
| Beijing Eagle Sky Pharmatech Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (10) 5979-9429 8875-5821 | |||
 |
sophia_818@126.com contact@eagleskypharmatech.com | |||
 |
QQ chat | |||
| Chemical manufacturer since 2009 | ||||
| chemBlink standard supplier since 2010 | ||||
| Taizhou Tongxin Biopharmaceutical Technology Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 18652728585 | |||
 |
sales@allyrise.com | |||
 |
QQ chat | |||
| Chemical manufacturer since 2013 | ||||
| chemBlink standard supplier since 2013 | ||||
| Caerulum Pharma Discovery Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (21) 6455-6180 +86 189149758185 | |||
 |
sales-cpd@caerulumpharma.com | |||
 |
Skype Chat | |||
 |
QQ chat | |||
| Chemical manufacturer since 2012 | ||||
| chemBlink standard supplier since 2019 | ||||
| Classification | Organic raw materials >> Nitrile compound |
|---|---|
| Name | (1S)-1-Amino-2,3-dihydro-1H-indene-4-carbonitrile hydrochloride (1:1) |
| Synonyms | (S)-1-Amino-2,3-dihydro-1H-indene-4-carbonitrile hydrochloride |
| Molecular Structure | 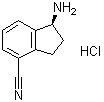 |
| Molecular Formula | C10H10N2.HCl |
| Molecular Weight | 194.66 |
| CAS Registry Number | 1306763-57-4 |
| EC Number | 865-129-4 |
| SMILES | C1CC2=C(C=CC=C2[C@H]1N)C#N.Cl |
| Hazard Symbols |
 GHS07 Warning Details
GHS07 Warning Details | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard Statements | H302-H315-H319-H335 Details | ||||||||||||||||||||||||
| Precautionary Statements | P261-P264-P264+P265-P270-P271-P280-P301+P317-P302+P352-P304+P340-P305+P351+P338-P319-P321-P330-P332+P317-P337+P317-P362+P364-P403+P233-P405-P501 Details | ||||||||||||||||||||||||
| Hazard Classification | |||||||||||||||||||||||||
| |||||||||||||||||||||||||
| SDS | Available | ||||||||||||||||||||||||
|
(1S)-1-Amino-2,3-dihydro-1H-indene-4-carbonitrile hydrochloride (1:1) is a notable chemical compound that has attracted attention in both academic research and industrial applications. The compound belongs to the class of indene derivatives, which are cyclic compounds that feature a benzene ring fused to a five-membered ring structure. The indene core is a key structural motif in many biologically active molecules, and the addition of an amino and a nitrile group to the structure enhances its chemical reactivity and potential utility. The discovery of (1S)-1-Amino-2,3-dihydro-1H-indene-4-carbonitrile hydrochloride stemmed from the ongoing exploration of indene-based compounds for their diverse biological activities. The compound was synthesized through a well-established synthetic route that involves the introduction of an amino group at the 1-position of the indene ring and a nitrile group at the 4-position. This specific arrangement results in a compound with distinctive electronic properties that make it useful for a variety of applications, particularly in medicinal chemistry. One of the main applications of (1S)-1-Amino-2,3-dihydro-1H-indene-4-carbonitrile hydrochloride is in the development of pharmaceutical agents. The compound's unique structure allows it to interact with various biological targets, and it has been investigated for its potential as a precursor in the synthesis of bioactive molecules. The amino group provides a site for further chemical modifications, which can be used to optimize the compound's affinity for specific receptors or enzymes. The nitrile group also contributes to the compound's reactivity, making it useful in drug discovery efforts focused on enzyme inhibition or receptor modulation. In addition to its pharmaceutical applications, (1S)-1-Amino-2,3-dihydro-1H-indene-4-carbonitrile hydrochloride has been studied for its potential as a building block in the synthesis of advanced materials. The indene core, known for its stability and versatility, can be incorporated into polymeric systems to improve their mechanical properties, such as strength and flexibility. The compound may also be used in the synthesis of functional materials for electronics or sensors, where its structural features can influence the properties of the final product. Further exploration of (1S)-1-Amino-2,3-dihydro-1H-indene-4-carbonitrile hydrochloride and its derivatives is ongoing, with research focusing on optimizing its pharmacological profile and expanding its range of applications. Studies are also examining the compound's potential for use in organic electronics, where its ability to form stable complexes and interact with other materials could open new avenues for device fabrication and performance enhancement. References 2020. Ozanimod. Pharmaceutical Substances. URL: https://pharmaceutical-substances.thieme.com/ps/search-results?docUri=KD-15-0081 |
| Market Analysis Reports |
| List of Reports Available for (1S)-1-Amino-2,3-dihydro-1H-indene-4-carbonitrile hydrochloride (1:1) |