Online Database of Chemicals from Around the World
| Antimex Pharmaceuticals & Chemicals Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (21) 5056-3169 | |||
 |
info@antimex.com | |||
| Chemical manufacturer | ||||
| chemBlink standard supplier since 2007 | ||||
| Simagchem Corporation | China | Inquire | ||
|---|---|---|---|---|
 |
+86 13806087780 | |||
 |
sale@simagchem.com | |||
| Chemical manufacturer since 2002 | ||||
| chemBlink standard supplier since 2008 | ||||
| Hefei TNJ Chemical Industry Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (551) 6541-8684 | |||
 |
sales@tnjchem.com | |||
| Chemical manufacturer since 2001 | ||||
| chemBlink standard supplier since 2010 | ||||
| BOC Sciences | USA | Inquire | ||
|---|---|---|---|---|
 |
+1 (631) 485-4226 | |||
 |
info@bocsci.com | |||
| Chemical manufacturer | ||||
| chemBlink standard supplier since 2010 | ||||
| Shanghai Fuqi Industrial & Trading Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (21) 6631-0933 | |||
 |
fqchem@126.com | |||
| Chemical distributor since 2002 | ||||
| chemBlink standard supplier since 2013 | ||||
| Uniwin Chemical Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (771) 576-9646 | |||
 |
tiffany@uniwinchemical.com | |||
| Chemical distributor since 2013 | ||||
| chemBlink standard supplier since 2013 | ||||
| Hangzhou Leap Chem Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (571) 8771-1850 | |||
 |
market19@leapchem.com | |||
 |
QQ chat | |||
| Chemical manufacturer since 2006 | ||||
| chemBlink standard supplier since 2015 | ||||
| Shanghai Haohong Biomedical Technology Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 4008210725 | |||
 |
malulu@leyan.com | |||
 |
QQ chat | |||
| Chemical manufacturer since 2011 | ||||
| chemBlink standard supplier since 2022 | ||||
| Classification | Organic raw materials >> Organometallic compound >> Organic sodium |
|---|---|
| Name | Ethylenediaminetetraacetic acid disodium salt |
| Synonyms | Edetic acid disodium salt; EDTA disodium salt; (Ethylenedinitrilo)tetraacetic acid disodium salt |
| Molecular Structure | 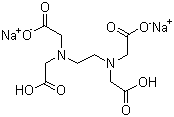 |
| Molecular Formula | C10H14N2Na2O8 |
| Molecular Weight | 336.21 |
| CAS Registry Number | 139-33-3 |
| EC Number | 205-358-3 |
| SMILES | C(CN(CC(=O)O)CC(=O)[O-])N(CC(=O)O)CC(=O)[O-].[Na+].[Na+] |
| Melting point | 237-245 ºC |
|---|---|
| Hazard Symbols |

 GHS07;GHS09 Warning Details
GHS07;GHS09 Warning Details | ||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard Statements | H302-H312-H315-H319-H332-H335-H373-H412 Details | ||||||||||||||||||||||||||||||||||||||||||||
| Precautionary Statements | P260-P261-P264-P264+P265-P270-P271-P273-P280-P301+P317-P302+P352-P304+P340-P305+P351+P338-P317-P319-P321-P330-P332+P317-P337+P317-P362+P364-P403+P233-P405-P501 Details | ||||||||||||||||||||||||||||||||||||||||||||
| Hazard Classification | |||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||
| SDS | Available | ||||||||||||||||||||||||||||||||||||||||||||
|
Ethylenediaminetetraacetic acid disodium salt, commonly abbreviated as EDTA-Na2, was first synthesized by German chemist Ferdinand M�nz in the early 20th century. Its discovery stemmed from the development of chelating agents capable of sequestering metal ions in solution. EDTA-Na2 is a derivative of ethylenediaminetetraacetic acid (EDTA) with enhanced water solubility and stability, making it a versatile compound with applications in various industries. EDTA-Na2 is widely used as a chelating agent in industrial processes and chemical analysis to sequester metal ions and prevent their interference or precipitation. It forms stable complexes with divalent and trivalent metal ions such as calcium, magnesium, iron, and copper, effectively binding them and inhibiting their catalytic or deleterious effects in solution. EDTA-Na2 is used in metal cleaning, textile processing, water treatment, and metal ion analysis. EDTA-Na2 is used in water treatment formulations to complex metal ions and prevent scaling or precipitation in water systems. It is added to boiler water treatment chemicals, detergents, and cleaners to chelate calcium and magnesium ions, thereby reducing water hardness and preventing scaling on surfaces and equipment. EDTA-Na2 improves water quality and extends the life of industrial equipment and pipes. EDTA-Na2 is used as a food additive in the food and beverage industry to prevent discoloration, maintain color stability, and preserve product quality. It acts as a chelating agent to sequester catalytic oxidation reactions and degrade food pigments or metal ions. EDTA-Na2 is added to canned fruits, vegetables, and beverages to extend shelf life and prevent spoilage due to metal-catalyzed oxidation. EDTA-Na2 is added to pharmaceutical formulations as a stabilizer and preservative to enhance drug stability and prevent degradation caused by metal ions or oxidation processes. It is used in parenteral solutions, ophthalmic preparations, and liquid dosage forms to chelate trace metal impurities and maintain drug efficacy and safety. EDTA-Na2 ensures the stability and integrity of drug products during storage and administration. EDTA-Na2 is used as a chelating agent and preservative in personal care products such as shampoos, soaps, and cosmetics to enhance product stability and prevent rancidity or discoloration. It binds metal ions in water or products, minimizing their adverse effects on product quality and appearance. EDTA-Na2 improves the performance and shelf life of personal care formulations, ensuring consumer satisfaction. EDTA-Na2 is used in medical and clinical settings for its anticoagulant properties and ability to chelate calcium ions. It is used as an anticoagulant in blood collection tubes to prevent blood clotting during sample collection and storage. EDTA-Na2 is also used in chelation therapy to treat heavy metal poisoning and as a diagnostic agent for metal ion analysis in medical diagnostics and research. References 1969. Determination of iron in solutions containing iron complexes. Journal of Clinical Pathology. DOI: 10.1136/jcp.22.3.301 2024. Stabilization of an Aqueous Solution of a Liquid Micro Fertilizer Containing Trilon B as a Chelating Agent. Structure of the Molecular Crystal of Sodium Carbamide Ethylenediaminetetraacetate Hydrate NaH3L1�(H2N)2CO�H2O. Russian Journal of Coordination Chemistry. DOI: 10.1134/s1070328424600608 1969. Purification and properties of an exo-beta-D-1,3-glucanase from sea urchin eggs. Biochimica et Biophysica Acta (BBA) - Enzymology. DOI: 10.1016/0005-2744(69)90224-1 |
| Market Analysis Reports |
| List of Reports Available for Ethylenediaminetetraacetic acid disodium salt |