Online Database of Chemicals from Around the World
| Fujian Wolfa Biotechnology Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 18050950397 | |||
 |
jomin@wolfabio.com | |||
 |
WhatsApp: +86 18359103607 | |||
| Chemical manufacturer since 2023 | ||||
| chemBlink standard supplier since 2024 | ||||
| Classification | Organic raw materials >> Organometallic compound >> Organic scandium, tantalum, thallium, tungsten, antimony, lanthanum, lead, vanadium, molybdenum, chromium, ytterbium, etc. |
|---|---|
| Name | Bis(acetonitrile)tetracarbonylmolybdenum(0) |
| Molecular Structure | 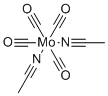 |
| Molecular Formula | C8H6N2O4Mo |
| Molecular Weight | 290.10 |
| CAS Registry Number | 14126-87-5 |
| SMILES | [Mo](C#[O])(C#[O])(C#[O])(C#[O])([N]#CC)[N]#CC |
|
Bis(acetonitrile)tetracarbonylmolybdenum(0) is a significant organometallic compound with valuable applications in catalysis and chemical synthesis. This substance, featuring molybdenum in a zero oxidation state and coordinated with carbon monoxide and acetonitrile ligands, has garnered interest due to its unique properties and versatility. The discovery of bis(acetonitrile)tetracarbonylmolybdenum(0) emerged from the exploration of transition metal carbonyl complexes. Molybdenum, a transition metal with variable oxidation states, is known for forming complexes with carbon monoxide, which stabilizes lower oxidation states. The synthesis of this compound involves reacting molybdenum hexacarbonyl with acetonitrile under controlled conditions, leading to the formation of the bis(acetonitrile)tetracarbonylmolybdenum(0) complex. The compound's structure is characterized by a central molybdenum atom surrounded by four carbon monoxide ligands and two acetonitrile ligands. The carbon monoxide ligands are crucial as they stabilize the molybdenum in its zero oxidation state, while the acetonitrile ligands contribute to the complex's solubility and reactivity. The resulting compound is a valuable intermediate for various chemical reactions due to its ability to participate in different catalytic processes. In catalysis, bis(acetonitrile)tetracarbonylmolybdenum(0) is utilized as a catalyst or catalyst precursor in several reactions. Its low oxidation state and the electron-donating nature of the carbon monoxide and acetonitrile ligands make it an effective catalyst in olefin metathesis and hydrocarbon functionalization. In particular, the compound has been employed in the hydrodesulfurization of thiophene derivatives, a critical process in refining and petrochemical industries. The compound also plays a role in organic synthesis, where it is used as a reagent in various transformations. For instance, it has been applied in the synthesis of molybdenum-based organometallic compounds that serve as intermediates for further chemical modifications. Its ability to stabilize molybdenum in a zero oxidation state facilitates the formation of new bonds and the activation of small molecules. Moreover, bis(acetonitrile)tetracarbonylmolybdenum(0) has been studied for its potential applications in material science. The compound's coordination chemistry allows for the design of novel materials with specific electronic and optical properties. It can be used to prepare molybdenum-based polymers and materials with unique characteristics, such as high thermal stability and conductivity. In summary, bis(acetonitrile)tetracarbonylmolybdenum(0) is a versatile organometallic compound with significant applications in catalysis, organic synthesis, and material science. Its unique structure and reactivity make it a valuable tool in advancing chemical processes and developing new materials. |
| Market Analysis Reports |
| List of Reports Available for Bis(acetonitrile)tetracarbonylmolybdenum(0) |