Online Database of Chemicals from Around the World
| Shanghai Daji Medical Technology Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 13816245196 | |||
 |
sales1@djpharm.com | |||
| Chemical distributor since 2019 | ||||
| chemBlink standard supplier since 2025 | ||||
| Classification | Organic raw materials >> Nitrile compound |
|---|---|
| Name | 5-Fluorobenzofuran-4-carbonitrile |
| Synonyms | 5-fluoro-1-benzofuran-4-carbonitrile |
| Molecular Structure | 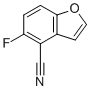 |
| Molecular Formula | C9H4FNO |
| Molecular Weight | 161.13 |
| CAS Registry Number | 1427446-97-6 |
| SMILES | C1=CC(=C(C2=C1OC=C2)C#N)F |
| Density | 1.3±0.1 g/cm3 Calc.* |
|---|---|
| Boiling point | 260.4±20.0 ºC 760 mmHg (Calc.)* |
| Flash point | 111.3±21.8 ºC (Calc.)* |
| Index of refraction | 1.599 (Calc.)* |
| * | Calculated using Advanced Chemistry Development (ACD/Labs) Software. |
| Hazard Symbols |
 GHS07 Warning Details
GHS07 Warning Details |
|---|---|
| Hazard Statements | H302-H315-H319 Details |
| Precautionary Statements | P501-P270-P264-P280-P302+P352-P337+P313-P305+P351+P338-P362+P364-P332+P313-P301+P312+P330 Details |
| SDS | Available |
|
5-Fluorobenzofuran-4-carbonitrile is a member of the benzofuran family, a group of bicyclic aromatic compounds consisting of a fused benzene ring and a furan ring. Benzofuran itself was first reported in the late nineteenth century during investigations into coal tar constituents, and numerous substituted benzofurans have since been prepared and examined in academic research and industrial development. Introduction of substituents such as halogens and nitrile groups onto the benzofuran core has been a recurrent strategy in synthetic chemistry aimed at adjusting reactivity and enabling incorporation into larger molecular architectures. In this context, 5-fluorobenzofuran-4-carbonitrile represents a halogenated, nitrile-containing derivative designed to combine the electronic influence of fluorine with the functional versatility of the nitrile moiety. The compound features a fluorine atom attached to the benzene portion of the bicyclic system and a nitrile group bound directly to the furan ring. Fluorine substitution on aromatic rings was established as an important structural modification in the twentieth century, owing to the small atomic radius of fluorine, the strength of the carbon fluorine bond, and the resulting effects on electron distribution across an extended aromatic system. The nitrile group, characterized by a carbon nitrogen triple bond, is recognized as a chemically stable and synthetically adaptable functionality that can serve as a precursor to amides, amines, carboxylic acids, and other derivatized products through established organic transformations. Research into substituted benzofurans expanded significantly over the second half of the twentieth century alongside advances in heterocycle synthesis, transition metal catalysis, and halogenation methodologies. Studies focused on the preparation of fluorinated benzofurans illustrated the influence of ring fluorination on the behavior of the heterocycle in electrophilic and nucleophilic reactions. Parallel investigations into benzofuran carbonitriles documented the utility of the nitrile functional group for subsequent synthetic elaboration under controlled conditions. Within this broader scientific background, 5-fluorobenzofuran-4-carbonitrile has been investigated as a synthetically useful intermediate that enables access to more complex benzofuran derivatives by transformation of the nitrile group or substitution at other positions of the aromatic system. Applications of benzofuran derivatives span fragrances, dyes, and materials research, reflecting the stability of the bicyclic framework and its optical and electronic characteristics. Halogenated benzofurans, including fluorinated members, have been examined for their physical properties and for their potential roles as building blocks in advanced materials. The incorporation of fluorine can influence melting behavior, vapor pressure, and compatibility with polymer matrices, while maintaining the rigidity of the benzofuran scaffold. Nitrile functional groups, in turn, are routinely used as connectors in the multistep synthesis of target molecules designed for materials or fine-chemical applications. The study of compounds such as 5-fluorobenzofuran-4-carbonitrile has also been closely linked to progress in analytical characterization. Methods developed for substituted benzofurans include chromatography for purification, infrared spectroscopy for identification of the nitrile stretch, and nuclear magnetic resonance spectroscopy for verification of aromatic substitution patterns. These techniques have been applied to confirm structural integrity, monitor reaction progress, and assess purity in synthetic sequences in which benzofuran carbonitriles serve as intermediates. Investigations into this compound and its analogues have highlighted the relationship between substitution pattern and chemical reactivity within the benzofuran series. The electron withdrawing effect of the nitrile group and the inductive influence of fluorine have been studied in the context of reactions that proceed through aromatic substitution or heterocycle functionalization. Findings from such studies have contributed to the development of reliable synthetic routes to multifunctional benzofuran derivatives and to the broader understanding of structure reactivity relationships in fused aromatic heterocycles. As part of continuing research into functionalized benzofurans, 5-fluorobenzofuran-4-carbonitrile is regarded as a structurally defined starting point for the preparation of more elaborate compounds. Its combination of a stable aromatic core, a strategically positioned halogen, and a convertible nitrile group underpins its value in planned synthetic sequences directed at applications in the fine chemicals sector and in exploratory materials science. References 2021. Synthesis of EEDi-5273. Synfacts, 17(12). DOI: 10.1055/s-0041-1737092 |
| Market Analysis Reports |
| List of Reports Available for 5-Fluorobenzofuran-4-carbonitrile |