Online Database of Chemicals from Around the World
| Sichuan Taienkang Pharmaceutical Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 15102825326 | |||
 |
sales@taienkangpharma.com | |||
 |
Skype Chat | |||
 |
QQ chat | |||
| Chemical manufacturer since 2020 | ||||
| chemBlink standard supplier since 2024 | ||||
| Classification | Pharmaceutical intermediate >> Heterocyclic compound intermediate >> Pyrimidine compound >> Ketones |
|---|---|
| Name | (4-Fluorophenyl)(2-(piperazin-1-yl)pyrimidin-5-yl)methanone |
| Molecular Structure | 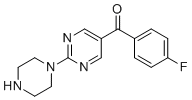 |
| Molecular Formula | C15H15FN4O |
| Molecular Weight | 286.30 |
| CAS Registry Number | 1703794-75-5 |
| SMILES | C1CN(CCN1)C2=NC=C(C=N2)C(=O)C3=CC=C(C=C3)F |
|
(4-Fluorophenyl)(2-(piperazin-1-yl)pyrimidin-5-yl)methanone is a chemical compound that has drawn attention in medicinal chemistry due to its potential as a bioactive molecule. The compound consists of a fluorophenyl group attached to a pyrimidine ring, which in turn is linked to a piperazine group via a methanone functionality. This structural configuration imparts distinct chemical properties that make it suitable for a range of applications, particularly in drug design. The discovery of (4-fluorophenyl)(2-(piperazin-1-yl)pyrimidin-5-yl)methanone was motivated by the growing interest in pyrimidine derivatives as potential therapeutic agents. Pyrimidines are well known for their biological activity, and when functionalized with groups such as piperazine and fluorophenyl, the compounds often exhibit enhanced pharmacological profiles. The presence of the piperazine moiety is particularly significant, as it can interact with various receptors, influencing neurochemical and cellular processes. Synthesis of (4-fluorophenyl)(2-(piperazin-1-yl)pyrimidin-5-yl)methanone is typically achieved through standard methods in organic chemistry, such as nucleophilic substitution or condensation reactions. The process generally involves the introduction of the piperazine group to a suitable pyrimidine precursor, followed by the addition of the fluorophenyl and methanone groups. The result is a compound with a high degree of chemical stability, which can be further modified for different applications. The compound has shown particular promise in drug development, especially in the area of neuropharmacology. Its piperazine component suggests potential interactions with neurotransmitter systems, such as serotonin or dopamine receptors, which are key targets in the treatment of psychiatric disorders like depression, anxiety, and schizophrenia. Additionally, the fluorophenyl group may enhance the compound’s lipophilicity, aiding in its ability to cross biological membranes and reach target sites more effectively. Beyond its therapeutic potential, (4-fluorophenyl)(2-(piperazin-1-yl)pyrimidin-5-yl)methanone may also be useful in the development of diagnostic tools or imaging agents. Its unique structure could enable its use in molecular probes that target specific biological pathways, providing valuable information in research and clinical settings. |
| Market Analysis Reports |
| List of Reports Available for (4-Fluorophenyl)(2-(piperazin-1-yl)pyrimidin-5-yl)methanone |