Online Database of Chemicals from Around the World
| Hefei TNJ Chemical Industry Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (551) 6541-8684 | |||
 |
sales@tnjchem.com | |||
| Chemical manufacturer since 2001 | ||||
| chemBlink standard supplier since 2010 | ||||
| Langfang GreatAp Chemicals Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (316) 209-8955 +86 18630626679 | |||
 |
sales@grechembld.com | |||
 |
QQ chat | |||
| Chemical manufacturer since 2001 | ||||
| chemBlink standard supplier since 2012 | ||||
| Earlier HK Company Limited | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (10) 6142-6769 +86 18616907430 | |||
 |
info@earlierchem.com | |||
 |
QQ chat | |||
| Chemical manufacturer since 2011 | ||||
| chemBlink standard supplier since 2013 | ||||
| Saian Chemical (shanghai) Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 18633110822 | |||
 |
teresa.liu@saianchem.com | |||
 |
QQ chat | |||
 |
WeChat: 18633110822 | |||
| Chemical distributor since 2018 | ||||
| chemBlink standard supplier since 2025 | ||||
| Classification | Inorganic chemical industry >> Inorganic salt >> Boride, borate and perborate |
|---|---|
| Name | Zinc Borohydride |
| Synonyms | Bis(tetrahydroborato)zinc; Zinc bis(tetrahydroborate); Zinc tetrahydroborate |
| Molecular Structure | 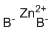 |
| Molecular Formula | B2H8Zn |
| Molecular Weight | 95.06 |
| CAS Registry Number | 17611-70-0 |
| EC Number | 810-074-3 |
| SMILES | [B-].[B-].[Zn+2] |
| Hazard Symbols |

 GHS05;GHS06 Danger Details
GHS05;GHS06 Danger Details | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard Statements | H301-H314 Details | ||||||||||||||||
| Precautionary Statements | P260-P264-P270-P280-P301+P316-P301+P330+P331-P302+P361+P354-P304+P340-P305+P354+P338-P316-P321-P330-P363-P405-P501 Details | ||||||||||||||||
| Hazard Classification | |||||||||||||||||
| |||||||||||||||||
|
Zinc borohydride is an inorganic compound composed of zinc and borohydride ions, typically represented by the formula Zn(BH4)2. It is part of the metal borohydride family, which consists of complexes formed between metal cations and the borohydride anion (BH4−). Zinc borohydride is a colorless to pale yellow solid that is sensitive to air and moisture, requiring careful handling under inert atmosphere conditions. The compound was first reported in the mid-20th century as part of efforts to explore metal borohydrides beyond the well-known lithium and sodium derivatives. Its preparation commonly involves the reaction of zinc salts, such as zinc chloride, with an excess of a borohydride salt like sodium borohydride in an aprotic solvent. This reaction yields zinc borohydride as a solid or solution, depending on conditions, and the product is often isolated under inert atmosphere to prevent decomposition. Zinc borohydride exhibits strong reducing properties, attributable to the hydride ions coordinated to the zinc center. This reducing ability is utilized in organic synthesis for selective transformations. It is capable of reducing aldehydes and ketones to the corresponding alcohols under milder conditions compared to other borohydrides, and it often shows greater selectivity in reductions involving sensitive functional groups. One significant advantage of zinc borohydride over more reactive hydrides like lithium aluminium hydride is its relatively lower reactivity with water and protic solvents, allowing for safer handling and milder reaction conditions. This makes it attractive in synthetic procedures where controlled reduction is required without harsh conditions that may degrade sensitive molecules. In addition to reductions, zinc borohydride has found applications as a catalyst or reagent in hydride transfer reactions. Its unique electronic environment due to the zinc ion influences the reactivity pattern, making it useful for specialized transformations in organic and organometallic chemistry. Zinc borohydride is also of interest in materials science, particularly in hydrogen storage research. Like other metal borohydrides, it has a high hydrogen content by weight and can release hydrogen gas upon thermal decomposition. Although the hydrogen release from zinc borohydride occurs at relatively high temperatures, its decomposition pathways and kinetics have been studied to understand the behavior of borohydride-based hydrogen storage materials better. Handling zinc borohydride requires precautions to avoid exposure to moisture and air, as it decomposes to release hydrogen gas and can ignite upon contact with water or air. It is generally stored and used under inert atmosphere conditions, such as nitrogen or argon. Appropriate personal protective equipment and working in a fume hood are standard safety practices. Zinc borohydride decomposes thermally to yield zinc metal or zinc-containing species and boron-hydrogen compounds. The decomposition is exothermic and must be controlled to prevent hazardous conditions. The compound is incompatible with oxidizing agents, acids, and water, and its disposal requires careful neutralization to avoid environmental and safety hazards. Overall, zinc borohydride is a valuable reagent in synthetic chemistry, especially for reductions requiring mild and selective conditions. Its unique properties among metal borohydrides continue to prompt research into its applications in both organic synthesis and hydrogen storage technologies. References 2007. Reduction of Cyclic Anhydrides to Lactones. Science of Synthesis. URL: https://science-of-synthesis.thieme.com/app/text/?id=SD-020-01412 2009. Using Zinc(II) Borohydride�Pyridine. Science of Synthesis. URL: https://science-of-synthesis.thieme.com/app/text/?id=SD-040-00081 2010. Using Zinc Borohydride. Science of Synthesis. URL: https://science-of-synthesis.thieme.com/app/text/?id=SD-041-00273 |
| Market Analysis Reports |
| List of Reports Available for Zinc Borohydride |