Online Database of Chemicals from Around the World
| Shanghai Daji Medical Technology Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 13816245196 | |||
 |
sales1@djpharm.com | |||
| Chemical distributor since 2019 | ||||
| chemBlink standard supplier since 2025 | ||||
| Classification | Organic raw materials >> Alcohols, phenols, phenolic compounds and derivatives |
|---|---|
| Name | 1,2-Di-O-dodecyl-rac-glycerol |
| Synonyms | 2,3-didodecoxypropan-1-ol |
| Molecular Structure | 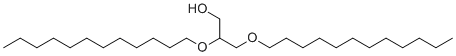 |
| Molecular Formula | C27H56O3 |
| Molecular Weight | 428.73 |
| CAS Registry Number | 17677-18-8 |
| SMILES | CCCCCCCCCCCCOCC(CO)OCCCCCCCCCCCC |
| Density | 0.9±0.1 g/cm3 Calc.* |
|---|---|
| Boiling point | 520.6±30.0 ºC 760 mmHg (Calc.)* |
| Flash point | 268.6±24.6 ºC (Calc.)* |
| Index of refraction | 1.457 (Calc.)* |
| * | Calculated using Advanced Chemistry Development (ACD/Labs) Software. |
|
1,2-Di-O-dodecyl-rac-glycerol is a glycerol-derived lipid in which the primary and secondary hydroxyl groups of glycerol are esterified or etherified with dodecyl chains, resulting in a racemic mixture of stereoisomers. This structure belongs to the class of dialkylglycerols, which are recognized for their amphiphilic character, combining a hydrophilic glycerol head with two hydrophobic alkyl chains. Such molecules have attracted attention for their utility in the study of membrane-mimetic systems, as intermediates in lipid chemistry, and in the design of bioactive lipid analogues. The synthesis of 1,2-di-O-dodecyl-rac-glycerol generally starts from glycerol or a protected glycerol derivative. Alkylation of the hydroxyl groups with dodecyl halides under basic conditions or using activated alkyl donors provides the dialkylated product. Protecting groups may be employed to achieve regioselectivity, ensuring that both the primary and secondary hydroxyl groups are substituted efficiently while avoiding over-alkylation or side reactions. Purification is typically achieved through column chromatography or recrystallization, yielding a colorless to pale solid or viscous liquid, depending on the degree of purity and the specific stereoisomer distribution. In synthetic organic chemistry, 1,2-di-O-dodecyl-rac-glycerol serves as a versatile intermediate for the preparation of ether lipids, glycolipids, and other lipid analogues. The dialkylglycerol scaffold can be further functionalized at the remaining hydroxyl group, or incorporated into more complex phospholipids and glycoglycerol derivatives. The racemic nature of the compound allows researchers to explore stereochemical effects in membrane models and in the synthesis of enantiomerically enriched bioactive lipids. In biological and medicinal research, dialkylglycerols are used to investigate membrane dynamics, lipid–protein interactions, and signal transduction pathways. Lipid analogues based on 1,2-di-O-dodecyl-rac-glycerol can serve as mimics of naturally occurring ether lipids, which are known to modulate enzyme activity, receptor binding, and cellular signaling. The amphiphilic character of the molecule facilitates incorporation into liposomes, micelles, and other supramolecular assemblies, making it useful in studies of drug delivery and membrane biophysics. The compound also has relevance in material science, particularly in the preparation of organized lipid films, vesicles, and nanostructured materials. Its combination of hydrophilic and hydrophobic components allows self-assembly into bilayers or other nanostructures, which can be exploited for encapsulation, controlled release, and surface functionalization applications. Modifying the chain length or stereochemistry of the glycerol backbone enables tuning of physical properties such as phase behavior, fluidity, and stability. Physically, 1,2-di-O-dodecyl-rac-glycerol is typically obtained as a viscous liquid or waxy solid with solubility in nonpolar or moderately polar organic solvents such as chloroform, dichloromethane, and ethanol. It is stable under standard laboratory conditions but should be stored away from strong acids, bases, and oxidizing agents to prevent degradation of the alkyl chains. Proper storage ensures the compound remains suitable for synthetic, biophysical, and materials applications. Overall, 1,2-di-O-dodecyl-rac-glycerol is a racemic dialkylglycerol featuring two dodecyl chains linked to a glycerol backbone. Its amphiphilic nature, chemical versatility, and capacity for further functionalization make it a valuable intermediate in lipid synthesis, a tool for studying membrane systems, and a building block for bioactive and material applications. References 2017. Development and Optimization of a High-Throughput Screening Assay for Rapid Evaluation of Lipstatin Production by Streptomyces Strains. Current Microbiology, 75(6). DOI: 10.1007/s00284-017-1420-x 1994. A comparison of different strategies for lipase-catalyzed synthesis of partial glycerides. Biotechnology Letters, 16(7). DOI: 10.1007/bf00136474 |
| Market Analysis Reports |
| List of Reports Available for 1,2-Di-O-dodecyl-rac-glycerol |