Online Database of Chemicals from Around the World
| Epochem Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (21) 6760-1595 6760-1597 | |||
 |
seth_wang@epochem.com | |||
 |
QQ chat | |||
| Chemical manufacturer since 2006 | ||||
| chemBlink standard supplier since 2005 | ||||
| Jinan Chenghui-Shuangda Chemical Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (531) 5889-7051 +86 15053146086 | |||
 |
jnchsd@qq.com | |||
 |
QQ chat | |||
| Chemical manufacturer since 2002 | ||||
| chemBlink standard supplier since 2007 | ||||
| Beijing Reco Technology Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (10) 6060-5164 | |||
 |
sales@recotechnology.com | |||
 |
QQ chat | |||
| Chemical manufacturer | ||||
| chemBlink standard supplier since 2010 | ||||
| BOC Sciences | USA | Inquire | ||
|---|---|---|---|---|
 |
+1 (631) 485-4226 | |||
 |
info@bocsci.com | |||
| Chemical manufacturer | ||||
| chemBlink standard supplier since 2010 | ||||
| Qingdao Xinnuo Pharmaceutical Chemical Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (531) 6656-1050 +86 18615688656 +86 18653156686 | |||
 |
sales@xinnuopharma.com | |||
 |
Skype Chat | |||
 |
QQ chat | |||
| Chemical manufacturer since 2013 | ||||
| chemBlink standard supplier since 2014 | ||||
| Leap Chem Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (852) 3060-6658 | |||
 |
market19@leapchem.com | |||
 |
QQ chat | |||
| Chemical manufacturer since 2006 | ||||
| chemBlink standard supplier since 2015 | ||||
| Typu Group | China | Inquire | ||
|---|---|---|---|---|
 |
+86 85220966 | |||
 |
order@typugroup.com | |||
| Chemical distributor since 2008 | ||||
| chemBlink standard supplier since 2021 | ||||
| Demeikai (hongkong)biotechnology Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 18942921723 | |||
 |
wzh@dmksw.xin | |||
 |
Skype Chat | |||
| Chemical distributor since 2017 | ||||
| chemBlink standard supplier since 2021 | ||||
| Classification | API >> Anesthetic agents >> Local anesthetics |
|---|---|
| Name | Prilocaine hydrochloride |
| Synonyms | Propitocaine hydrochloride; 2-(Propylamino)-o-propionotoluidide hydrochloride; N-(2-Methylphenyl)-2-(propylamino)-propanamide hydrochloride |
| Molecular Structure | 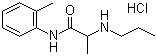 |
| Protein Sequence | A |
| Molecular Formula | C13H20N2O.HCl |
| Molecular Weight | 256.77 |
| CAS Registry Number | 1786-81-8 |
| EC Number | 217-244-0 |
| SMILES | CCCNC(C)C(=O)NC1=CC=CC=C1C.Cl |
| Melting point | 168 - 170 ºC (Expl.) |
|---|---|
| Solubility | 10 mM (DMSO) (Expl.) |
| Hazard Symbols |
 GHS07 Warning Details
GHS07 Warning Details | ||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard Statements | H302-H312-H315-H319-H412 Details | ||||||||||||||||||||||||||||||||
| Precautionary Statements | P264-P264+P265-P270-P273-P280-P301+P317-P302+P352-P305+P351+P338-P317-P321-P330-P332+P317-P337+P317-P362+P364-P501 Details | ||||||||||||||||||||||||||||||||
| Hazard Classification | |||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||
| SDS | Available | ||||||||||||||||||||||||||||||||
|
Prilocaine hydrochloride is an amide-type local anesthetic commonly used to induce regional anesthesia in medical and dental procedures. It functions by reversibly blocking voltage-gated sodium channels in nerve cell membranes, preventing the initiation and conduction of nerve impulses, which results in a temporary loss of sensation in the targeted area. Prilocaine is characterized by a rapid onset of action and an intermediate duration of anesthesia, making it suitable for procedures requiring moderate-length nerve blockade. Prilocaine was first synthesized in the mid-1950s and introduced into clinical use shortly thereafter as part of efforts to develop amide local anesthetics with improved safety profiles and clinical efficacy compared to earlier esters. It is chemically related to lidocaine but differs structurally by the presence of an additional methyl group, which influences its pharmacokinetic and pharmacodynamic properties. The hydrochloride salt form of prilocaine enhances its solubility and stability for use in injectable formulations. It is commonly provided in concentrations such as 0.5%, 1%, or 2%, and can be used alone or in combination with vasoconstrictors like epinephrine to prolong its anesthetic effect and reduce systemic absorption. Prilocaine exhibits relatively low vasodilatory activity, which contributes to a longer duration of action in comparison to some other local anesthetics. Clinically, prilocaine hydrochloride is widely used in dental anesthesia, peripheral nerve blocks, infiltration anesthesia, and spinal anesthesia. It is also employed in topical formulations for dermal anesthesia, often combined with lidocaine in eutectic mixtures to facilitate skin penetration for minor surgical or diagnostic procedures. Its rapid onset and intermediate duration make it particularly useful in outpatient and ambulatory care settings. Pharmacokinetically, prilocaine is absorbed quickly from the site of administration, and it undergoes hepatic metabolism primarily via amidases to produce inactive metabolites. A notable metabolite is ortho-toluidine, which in large amounts can cause methemoglobinemia—a condition in which hemoglobin is oxidized to methemoglobin and is unable to carry oxygen efficiently. However, methemoglobinemia is uncommon at therapeutic doses, and clinical guidelines recommend caution or dose adjustment in patients at higher risk, such as infants, patients with preexisting anemia, or those taking oxidizing drugs. Prilocaine has an elimination half-life of approximately 1.6 to 2 hours, with renal excretion of metabolites. Its systemic toxicity profile is similar to other amide local anesthetics, with adverse effects potentially involving central nervous system excitation (e.g., dizziness, tinnitus, seizures) and cardiovascular effects (e.g., hypotension, bradycardia) when overdosed or inadvertently injected intravascularly. Nevertheless, prilocaine is generally well tolerated when administered properly. In comparison to lidocaine, prilocaine is considered to have slightly less vasodilatory effect, which can contribute to longer anesthesia duration without vasoconstrictors. It also has a lower incidence of systemic toxicity. However, the risk of methemoglobinemia distinguishes it clinically and requires appropriate monitoring and dosing considerations. In summary, prilocaine hydrochloride is a widely used amide local anesthetic with rapid onset and intermediate duration of action. Its chemical and pharmacological properties make it suitable for various anesthetic techniques, including infiltration, nerve block, spinal, and topical anesthesia. Its safety profile and effectiveness have been well established in clinical practice, making it an important agent in regional anesthesia and pain management. References 1990. Regional variations in analgesic efficacy of EMLA cream. Quantitatively evaluated by argon laser stimulation. Acta Dermato-Venereologica, 70(4). DOI: 10.2340/0001555570314318 1998. Transient neurologic symptoms after spinal anesthesia: a lower incidence with prilocaine and bupivacaine than with lidocaine. Anesthesiology, 88(3). DOI: 10.1097/00000542-199803000-00012 2010. Safety of Lidocaine 15% and Prilocaine 5% Topical Ointment Used as Local Anesthesia for Intense Pulsed Light Treatment. Dermatologic Surgery, 36(7). DOI: 10.1111/j.1524-4725.2010.01597.x |
| Market Analysis Reports |
| List of Reports Available for Prilocaine hydrochloride |