Online Database of Chemicals from Around the World
| Changzhou Fluoride Chemical Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (519) 8989-2883 +86 15995021858 | |||
 |
ycyvip@gmail.com ycyvip@hotmail.com | |||
 |
QQ chat | |||
| Chemical manufacturer | ||||
| chemBlink standard supplier since 2008 | ||||
| Quzhou HuntBio Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (571) 6406-7827 +86 13868119375 | |||
 |
sales@huntchem.com | |||
 |
QQ chat | |||
| Chemical manufacturer since 2017 | ||||
| chemBlink standard supplier since 2009 | ||||
| Novasyn Organics Pvt. Ltd. | India | Inquire | ||
|---|---|---|---|---|
 |
+91 (406) 620-2233 633-9111 | |||
 |
info@novasynorganics.com | |||
| Chemical manufacturer | ||||
| chemBlink standard supplier since 2010 | ||||
| Wilshire Technologies, Inc. | USA | Inquire | ||
|---|---|---|---|---|
 |
+1 (609) 683-1117 | |||
 |
Wilshire-info@evonik.com | |||
| Chemical manufacturer since 1997 | ||||
| chemBlink standard supplier since 2010 | ||||
| SL Drugs and Pharmaceuticals Pvt. Ltd. | India | Inquire | ||
|---|---|---|---|---|
 |
+91 (40) 6661-1133 | |||
 |
enquiry@sldrugs.com | |||
| Chemical distributor since 1999 | ||||
| chemBlink standard supplier since 2010 | ||||
| Hangzhou Leap Chem Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (571) 8771-1850 | |||
 |
market19@leapchem.com | |||
 |
QQ chat | |||
| Chemical manufacturer since 2006 | ||||
| chemBlink standard supplier since 2015 | ||||
| Amadis Chemical Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (571) 8992-5085 | |||
 |
sales@amadischem.com | |||
| Chemical manufacturer since 2010 | ||||
| chemBlink standard supplier since 2015 | ||||
| Neostar United (Changzhou) Industrial Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (519) 8555-7386 +86 18015025600 | |||
 |
marketing1@neostarunited.com | |||
| Chemical distributor since 2014 | ||||
| chemBlink standard supplier since 2020 | ||||
| Classification | Pharmaceutical intermediate >> Heterocyclic compound intermediate >> Pyridine compound >> Aminopyridine |
|---|---|
| Name | 4-Amino-2-picoline |
| Synonyms | 4-Amino-2-methylpyridine |
| Molecular Structure | 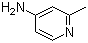 |
| Molecular Formula | C6H8N2 |
| Molecular Weight | 108.14 |
| CAS Registry Number | 18437-58-6 |
| EC Number | 625-782-6 |
| SMILES | CC1=NC=CC(=C1)N |
| Melting point | 93-97 ºC |
|---|---|
| Hazard Symbols |

 GHS05;GHS07 Danger Details
GHS05;GHS07 Danger Details | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard Statements | H302-H315-H318-H335 Details | ||||||||||||||||||||||||||||||||||||
| Precautionary Statements | P261-P264-P264+P265-P270-P271-P280-P301+P317-P302+P352-P304+P340-P305+P354+P338-P317-P319-P321-P330-P332+P317-P362+P364-P403+P233-P405-P501 Details | ||||||||||||||||||||||||||||||||||||
| Hazard Classification | |||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||
| SDS | Available | ||||||||||||||||||||||||||||||||||||
|
4-Amino-2-picoline, a derivative of picoline, was first synthesized in the early 20th century as chemists explored the reactivity of pyridine derivatives. Picoline, a methylpyridine isomer, served as a starting material for various chemical reactions aimed at producing functionalized pyridine derivatives with diverse properties. The synthesis of 4-Amino-2-picoline involves the introduction of an amino group at the 4-position of the picoline ring, yielding a compound with distinct chemical and biological characteristics. 4-Amino-2-picoline serves as a valuable intermediate in the synthesis of pharmaceutical compounds. Its unique structure and reactivity make it an essential building block for the production of various drugs, including antihypertensives, antihistamines, and anti-inflammatory agents. Chemical modifications of the amino group or the pyridine ring enable the creation of drug candidates with improved efficacy, bioavailability, or selectivity. In the agricultural sector, 4-Amino-2-picoline finds application as a precursor for the synthesis of agrochemicals and pesticides. Pyridine derivatives possess pesticidal properties, and modifications of their structure can enhance their effectiveness against pests and weeds. By incorporating 4-Amino-2-picoline into pesticide formulations, researchers aim to develop products that can protect crops from damage caused by insects, fungi, and other pathogens. 4-Amino-2-picoline is utilized in the formulation of corrosion inhibitors, particularly for metal surfaces exposed to harsh environments. Its chemical properties enable it to form protective layers on metal surfaces, inhibiting corrosion caused by factors such as moisture, oxygen, and chemical pollutants. As a versatile chemical intermediate, 4-Amino-2-picoline plays a role in the production of dyestuffs and pigments. Its incorporation into dye molecules imparts specific color properties, making it valuable in the textile, printing, and coloring industries. Beyond its established applications, ongoing research explores new uses for 4-Amino-2-picoline in fields such as materials science, catalysis, and organic synthesis. Scientists investigate its reactivity and potential interactions with other molecules to develop innovative materials, catalysts, and chemical processes. References 1976 Synthesis of 6- and 8-nitroindolizines. Chemistry of Heterocyclic Compounds, 12(7). DOI: 10.1007/bf00477009 2011 High-throughput screening identification of poliovirus RNA-dependent RNA polymerase inhibitors. Antiviral Research, 92(1). DOI: 10.1016/j.antiviral.2011.06.006 2022 C-H Amination with Nucleophilic N-H Reagents. Science of Synthesis. URL: https://science-of-synthesis.thieme.com/app/text/?id=SD-238-00093 |
| Market Analysis Reports |
| List of Reports Available for 4-Amino-2-picoline |