Online Database of Chemicals from Around the World
| Shanghai Edu Chemical Technology Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 19301251526 | |||
 |
sales1@chemido.com | |||
 |
QQ chat | |||
| Chemical manufacturer since 2011 | ||||
| chemBlink standard supplier since 2025 | ||||
| Classification | Organic raw materials >> Ketone compound |
|---|---|
| Name | 1-(Methylamino)propan-2-one hydrochloride |
| Molecular Structure | 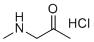 |
| Protein Sequence | G |
| Molecular Formula | C4H10ClNO |
| Molecular Weight | 123.58 |
| CAS Registry Number | 20041-74-1 |
| EC Number | 843-537-3 |
| SMILES | CC(=O)CNC.Cl |
| Hazard Symbols |
 GHS07 Warning Details
GHS07 Warning Details | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard Statements | H302-H315-H319-H335 Details | ||||||||||||||||||||||||
| Precautionary Statements | P261-P264-P264+P265-P270-P271-P280-P301+P317-P302+P352-P304+P340-P305+P351+P338-P319-P321-P330-P332+P317-P337+P317-P362+P364-P403+P233-P405-P501 Details | ||||||||||||||||||||||||
| Hazard Classification | |||||||||||||||||||||||||
| |||||||||||||||||||||||||
| SDS | Available | ||||||||||||||||||||||||
|
1-(Methylamino)propan-2-one hydrochloride is a small organic compound consisting of a methylamino group attached to the first carbon of a propan-2-one backbone, with the molecule isolated as its hydrochloride salt. The structure contains a ketone functional group at the second carbon and a primary methylamino substituent at the first carbon, forming a simple α-aminoketone motif. Compounds of this class have been studied for their reactivity and use as intermediates in synthetic organic chemistry, owing to the combination of a nucleophilic amino group and an electrophilic carbonyl center. The synthesis of 1-(methylamino)propan-2-one hydrochloride generally involves the reductive amination of the corresponding α-ketone with methylamine, followed by treatment with hydrochloric acid to isolate the hydrochloride salt. The procedure requires careful control of reaction conditions to prevent over-alkylation or side reactions, ensuring selective formation of the monomethylated aminoketone. Purification typically involves crystallization of the hydrochloride salt from suitable polar solvents to yield a solid product. In organic synthesis, this compound serves as a key intermediate for the preparation of substituted α-aminoketones, which can be further transformed into a variety of heterocycles, amides, and other functionalized molecules. The ketone group allows for nucleophilic addition, condensation, or reduction reactions, while the methylamino substituent can participate in alkylation or acylation reactions. This dual functionality provides flexibility for constructing more complex chemical architectures. The compound also finds utility in medicinal chemistry research as a scaffold for the development of biologically active molecules. α-Aminoketone derivatives are known to interact with enzymatic systems and receptor proteins through hydrogen bonding and electrostatic interactions. The methylamino group contributes to basicity and solubility, while the carbonyl moiety can participate in covalent or non-covalent interactions in biological systems, making the molecule suitable for incorporation into diverse pharmacophores. Physically, 1-(methylamino)propan-2-one hydrochloride is typically obtained as a crystalline solid with high solubility in water and polar organic solvents such as ethanol or methanol. It is generally stable under ambient conditions when stored in a tightly sealed container, but should be protected from strong oxidizing agents and extreme pH conditions that could modify either the amino or carbonyl functionality. Proper handling ensures the integrity of the compound for synthetic and research applications. Overall, 1-(methylamino)propan-2-one hydrochloride is a chemically versatile α-aminoketone hydrochloride featuring both a nucleophilic amino group and an electrophilic ketone. Its straightforward structure and dual reactivity make it a valuable intermediate for organic synthesis, functionalized molecule preparation, and medicinal chemistry investigations. References 2007. 2-Arylthiazole Derivatives as CXCR3 Receptor Modulators. WO Patent, 2007070433. |
| Market Analysis Reports |
| List of Reports Available for 1-(Methylamino)propan-2-one hydrochloride |