Online Database of Chemicals from Around the World
| Chemfun Medical Technology (shanghai) Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (21) 6722-0633 | |||
 |
sales1@chemfun.net | |||
 |
QQ chat | |||
 |
WeChat: 3046659337 | |||
| Chemical manufacturer since 2010 | ||||
| chemBlink standard supplier since 2010 | ||||
| Classification | Chemical reagent >> Organic reagent >> Amine salt (ammonium salt) |
|---|---|
| Name | (5-Fluoro-2,3-dihydrobenzofuran-4-yl)methanamine hydrochloride |
| Molecular Structure | 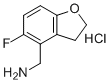 |
| Molecular Formula | C9H11ClFNO |
| Molecular Weight | 203.64 |
| CAS Registry Number | 2135600-87-0 |
| EC Number | 899-027-6 |
| SMILES | C1COC2=C1C(=C(C=C2)F)CN.Cl |
| Solubility | water: Soluble (0.735 mg/mL) Calc. |
|---|---|
| Hazard Symbols |
 GHS07 Warning Details
GHS07 Warning Details |
|---|---|
| Hazard Statements | H302-H315-H319-H335 Details |
| Precautionary Statements | P261-P264-P264+P265-P270-P271-P280-P301+P317-P302+P352-P304+P340-P305+P351+P338-P319-P321-P330-P332+P317-P337+P317-P362+P364-P403+P233-P405-P501 Details |
| SDS | Available |
|
(5-Fluoro-2,3-dihydrobenzofuran-4-yl)methanamine hydrochloride represents a member of a well-studied group of functionalized benzofuran derivatives, a class whose origins and applications have been documented across several decades of heterocyclic research. The benzofuran framework itself was identified in numerous natural products during early investigations into plant metabolites, where its structural features prompted extensive studies on synthetic accessibility and functional modification. Work on hydrogenated benzofurans subsequently clarified that partial saturation of the heterocyclic ring could be achieved without disrupting substitution patterns required for downstream transformations, establishing methods that later supported the preparation of amino- and halo-substituted derivatives. Research on the preparation of fluorinated benzofurans showed that selective introduction of fluorine atoms into aromatic and partially hydrogenated systems was feasible through electrophilic or nucleophilic fluorination routes. Studies demonstrated that substitution at positions comparable to the 5-position of the benzofuran motif could be controlled through directing group effects and ring electronics. These findings enabled the preparation of stable fluoro-substituted benzofurans suitable for further derivatization. Concurrently, the introduction of amine substituents onto benzofuran rings was explored through reduction of nitro intermediates, displacement reactions, or catalytic amination strategies. These methods became important for producing derivatives intended for use as synthetic intermediates in medicinal chemistry and related areas. Investigations into the reactivity of partially saturated benzofurans identified that the dihydrobenzofuran skeleton was compatible with palladium-catalyzed cross-coupling, metal-mediated annulation, and electrophilic aromatic substitution. These findings broadened the range of accessible substitution patterns and enabled construction of molecules with combinations of halogen and amine substituents. The ability to maintain structural integrity under standard synthetic conditions made these derivatives suitable for incorporation into multistep reaction sequences. Studies also demonstrated that halogen substituents on the benzofuran core could influence subsequent reactivity by altering oxidative addition rates in catalytic cycles, a feature relevant to the design of intermediates used in complex molecule synthesis. Applications of amino- and fluoro-substituted benzofurans were documented in research focused on structure–activity relationships of heterocyclic scaffolds. Aminomethyl substitution patterns, in particular, were explored as modulators of polarity and binding affinity in compounds under evaluation for biological activity. Fluorine incorporation was studied for its effects on metabolic stability, electronic distribution, and conformational bias, features that became widely recognized across research on fluorinated heterocycles. These investigations established that fluorinated benzofuran derivatives could serve as components in lead-optimization studies or as intermediates for generating analogs with defined physicochemical characteristics. Additional studies addressed the preparation of benzofuran derivatives with multiple substituents. Combinations of halogen and amine groups were shown to be compatible with conditions commonly used for cyclization methods such as palladium-catalyzed annulation of o-haloaryl substrates with alkynes. These reactions provided one of the most reliable routes to benzofuran cores and were later extended to produce partially hydrogenated variants. Techniques involving metal-catalyzed intramolecular hydroarylation of alkynes further expanded synthetic options and allowed access to substituted dihydrobenzofuran systems in high yields. As research progressed, various benzofuran derivatives, including those bearing halogen and amino substituents, were investigated for their chemical behavior, stability, and suitability as intermediates in the synthesis of more complex molecules. Their compatibility with nucleophilic substitution, reductive amination, and protective-group strategies supported broad utility in organic synthesis. Such derivatives were also included in studies of heterocyclic frameworks used to examine general principles of aromatic substitution, ring reactivity, and functional-group interplay. Through these developments, compounds of the structural type represented by (5-fluoro-2,3-dihydrobenzofuran-4-yl)methanamine hydrochloride came to occupy an established place among heterocyclic building blocks prepared and utilized according to methods validated in the literature. References 2024. A preparation method of (5-fluoro-2,3-dihydrobenzofuran-4-yl)methylamine hydrochloride. CN Patent, 119192106. 2022. Methods and compounds for modulating myotonic dystrophy 1. World Intellectual Property Organization. URL: WO-2022126000-A1 |
| Market Analysis Reports |
| List of Reports Available for (5-Fluoro-2,3-dihydrobenzofuran-4-yl)methanamine hydrochloride |