Online Database of Chemicals from Around the World
| Natural Micron Pharm Tech Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (0538) 535-2278 | |||
 |
info@nmpharmtech.com | |||
| Chemical manufacturer since 2012 | ||||
| chemBlink standard supplier since 2025 | ||||
| Classification | Flavors and spices >> Synthetic spice >> Phenols, ethers and epoxides >> Phenolic flavors |
|---|---|
| Name | Dihydrohonokiol |
| Synonyms | 2-(4-hydroxy-3-prop-2-enylphenyl)-4-propylphenol |
| Molecular Structure | 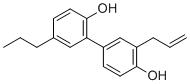 |
| Molecular Formula | C18H20O2 |
| Molecular Weight | 268.35 |
| CAS Registry Number | 219565-74-9 |
| SMILES | CCCC1=CC(=C(C=C1)O)C2=CC(=C(C=C2)O)CC=C |
| Hazard Symbols |

 GHS05;GHS09 Danger Details
GHS05;GHS09 Danger Details |
|---|---|
| Hazard Statements | H318-H411 Details |
| Precautionary Statements | P273-P280-P305+P351+P338+P310-P391-P501 Details |
| Transport Information | UN 3077 |
| SDS | Available |
|
Dihydrohonokiol is a neolignan compound derived from magnolia species, especially *Magnolia officinalis* and *Magnolia grandiflora*. It is structurally related to honokiol, a bioactive compound well known in traditional East Asian medicine. The primary distinction between dihydrohonokiol and honokiol lies in the saturation of the allyl side chains. While honokiol contains unsaturated allyl groups, dihydrohonokiol features hydrogenated propyl groups, making it more stable under certain conditions. The discovery of dihydrohonokiol dates back to phytochemical investigations of magnolia bark, which has long been used in traditional Chinese and Japanese herbal medicine, primarily for its anxiolytic, anti-inflammatory, and antimicrobial properties. The compound was isolated during studies focusing on the lignan content of magnolia bark and subsequently characterized using spectroscopic techniques such as nuclear magnetic resonance (NMR) and mass spectrometry (MS). These studies confirmed its structural identity as a biphenolic neolignan with two propyl-substituted aromatic rings joined via a central oxygen atom. In modern pharmacological research, dihydrohonokiol has gained attention for its neuroprotective and anxiolytic effects. Animal models have shown that it acts as a positive modulator of the γ-aminobutyric acid (GABA) receptor system, particularly the GABAA receptor. This mechanism is believed to underlie its potential as a natural anxiolytic agent. Unlike traditional benzodiazepines, dihydrohonokiol does not induce sedation at anxiolytic doses, making it a promising candidate for the development of safer treatments for anxiety disorders. Beyond its effects on the central nervous system, dihydrohonokiol has demonstrated significant antioxidant activity. Its biphenolic structure allows it to scavenge free radicals and inhibit lipid peroxidation, contributing to its protective effects in models of oxidative stress. Studies have also shown that it can inhibit the activation of nuclear factor kappa B (NF-κB), a transcription factor involved in the expression of pro-inflammatory cytokines. This suggests a broader anti-inflammatory profile for dihydrohonokiol, with potential applications in inflammatory diseases and neurodegenerative disorders. In the context of oncology, preliminary in vitro experiments have indicated that dihydrohonokiol may exert antiproliferative effects on various cancer cell lines. Its ability to induce apoptosis and inhibit angiogenesis has been observed, although further studies are needed to validate these effects in vivo and to elucidate the underlying molecular mechanisms. Nonetheless, these findings have sparked interest in neolignan derivatives as potential chemopreventive agents. Dihydrohonokiol’s pharmacokinetics and bioavailability remain subjects of ongoing investigation. Studies in rodents suggest that it can cross the blood-brain barrier, a crucial property for central nervous system-active agents. However, its relatively low water solubility may limit oral bioavailability, prompting research into formulation strategies such as nanoparticle encapsulation or chemical modification to enhance systemic absorption. Currently, dihydrohonokiol is not approved as a drug by major regulatory agencies, but it is available in some dietary supplements marketed for stress relief and cognitive support. Its inclusion in such products underscores the growing interest in phytochemicals as alternatives or complements to synthetic pharmaceuticals. However, the safety profile of long-term dihydrohonokiol use in humans is not fully established, and further clinical studies are required to determine optimal dosing, efficacy, and potential interactions. In conclusion, dihydrohonokiol represents a bioactive compound with diverse pharmacological properties rooted in traditional medicine and supported by modern research. Its anxiolytic, antioxidant, and anti-inflammatory activities make it a candidate of interest in the development of novel therapeutics, especially for neurological and inflammatory conditions. Further preclinical and clinical evaluations will be necessary to fully explore its therapeutic potential and to address questions related to safety, pharmacokinetics, and efficacy in human populations. References 2012. An expedient synthesis of honokiol and its analogues as potential neuropreventive agents. Bioorganic & Medicinal Chemistry Letters, 22(1). DOI: 10.1016/j.bmcl.2011.11.030 2005. Anxiolytic agent, dihydrohonokiol-B, recovers amyloid beta protein-induced neurotoxicity in cultured rat hippocampal neurons. Neuroscience Letters, 384(1-2). DOI: 10.1016/j.neulet.2005.04.081 2004. Efficient synthesis and structure-activity relationship of honokiol, a neurotrophic biphenyl-type neolignan. Bioorganic & Medicinal Chemistry Letters, 14(10). DOI: 10.1016/j.bmcl.2004.02.067 |
| Market Analysis Reports |
| List of Reports Available for Dihydrohonokiol |