Online Database of Chemicals from Around the World
| Shanghai Daji Medical Technology Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 13816245196 | |||
 |
sales1@djpharm.com | |||
| Chemical distributor since 2019 | ||||
| chemBlink standard supplier since 2025 | ||||
| Classification | Organic raw materials >> Ketone compound |
|---|---|
| Name | 4-(2-Acetyl-5-chlorophenyl)-5-methoxypyridin-2(1H)-one |
| Molecular Structure | 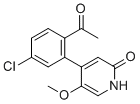 |
| Molecular Formula | C14H12ClNO3 |
| Molecular Weight | 277.70 |
| CAS Registry Number | 2201839-83-8 |
| SMILES | CC(=O)C1=C(C=C(C=C1)Cl)C2=CC(=O)NC=C2OC |
| Density | 1.3±0.1 g/cm3 Calc.* |
|---|---|
| Boiling point | 546.6±50.0 ºC 760 mmHg (Calc.)* |
| Flash point | 284.4±30.1 ºC (Calc.)* |
| Index of refraction | 1.606 (Calc.)* |
| * | Calculated using Advanced Chemistry Development (ACD/Labs) Software. |
| Hazard Symbols |
 GHS07 Warning Details
GHS07 Warning Details |
|---|---|
| Hazard Statements | H302-H315-H319-H335 Details |
| Precautionary Statements | P261-P264-P270-P271-P280-P301+P312-P302+P352-P304+P340-P305+P351+P338-P330-P332+P313-P337+P313-P362-P403+P233-P405-P501 Details |
| SDS | Available |
|
4-(2-Acetyl-5-chlorophenyl)-5-methoxypyridin-2(1H)-one is a heterocyclic compound consisting of a pyridin-2-one core substituted at the 4-position with a 2-acetyl-5-chlorophenyl group and at the 5-position with a methoxy group. The pyridin-2-one scaffold is a versatile bicyclic heterocycle known for its role in medicinal and synthetic chemistry, where the carbonyl and nitrogen atoms in the ring contribute to hydrogen bonding, tautomerism, and reactivity. Substitution with a chlorophenyl-acetyl group introduces both steric and electronic modifications, while the methoxy group at the 5-position influences electron density and solubility. The synthesis of 4-(2-acetyl-5-chlorophenyl)-5-methoxypyridin-2(1H)-one generally involves condensation of an appropriately substituted chlorophenyl ketone with a methoxypyridinone precursor. Key steps often include the formation of a C–C bond between the aromatic ketone and the pyridinone nucleus under controlled conditions, ensuring regioselectivity and preservation of sensitive functional groups. The methoxy substituent may be introduced prior to or after condensation, depending on the synthetic route. Purification is usually achieved via recrystallization or chromatographic separation to yield a solid product suitable for further chemical transformations. In organic synthesis, this compound functions as a valuable intermediate for the preparation of substituted pyridinone derivatives and more complex heterocyclic systems. The acetyl group provides a reactive site for nucleophilic addition, condensation, or enolate-based transformations, enabling the construction of extended conjugated systems or additional heterocycles. The methoxy group modulates electronic properties of the ring, influencing both reactivity and selectivity in subsequent functionalization steps. In medicinal chemistry, derivatives of 4-(2-acetyl-5-chlorophenyl)-5-methoxypyridin-2(1H)-one are explored for their potential as bioactive agents. The pyridinone core can engage in hydrogen bonding interactions with biological targets, while the chlorophenyl-acetyl substituent may contribute to hydrophobic interactions and influence binding affinity. The combination of electronic modulation from the methoxy and chloro substituents with the reactive acetyl site allows chemists to design and synthesize analogues for structure–activity relationship studies. The compound is also relevant in methodology development and chemical research. Its multifunctional nature—combining a pyridinone ring, a halogenated aromatic ketone, and a methoxy group—makes it suitable for studying selective C–C bond formation, electrophilic substitution, and functional group transformations. Researchers utilize it to explore regioselectivity, protective group strategies, and the synthesis of nitrogen-containing heterocycles for medicinal and material applications. Physically, 4-(2-acetyl-5-chlorophenyl)-5-methoxypyridin-2(1H)-one is typically obtained as a crystalline solid with moderate solubility in polar organic solvents such as ethanol, dichloromethane, and dimethylformamide. It is stable under standard laboratory conditions but should be protected from strong acids, bases, and oxidizing agents to preserve both the acetyl functionality and the pyridinone core. Proper storage ensures its suitability for synthetic and exploratory applications. Overall, 4-(2-acetyl-5-chlorophenyl)-5-methoxypyridin-2(1H)-one is a multifunctional heterocyclic intermediate featuring a pyridinone core with a chlorophenyl-acetyl substituent and a methoxy group. Its combination of reactive and electronically modulating functional groups allows selective chemical transformations and derivatization, making it a valuable building block in the synthesis of heterocycles, bioactive molecules, and complex synthetic targets in medicinal and organic chemistry. References 2023. Novel oxopyridine compound and its preparation method and use. CN Patent, 116262724. 2021. Medical application of FXIa inhibitor compound or salt thereof. CN Patent, 112675173. 2021. FXIa inhibitors and preparation method therefor and pharmaceutical use thereof. WO Patent, 2021057818. |
| Market Analysis Reports |
| List of Reports Available for 4-(2-Acetyl-5-chlorophenyl)-5-methoxypyridin-2(1H)-one |