Online Database of Chemicals from Around the World
| Hangzhou Verychem Science And Technology Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (571) 8816-2785 +86 13606544505 | |||
 |
lucy@verychem.com | |||
| Chemical manufacturer since 2004 | ||||
| chemBlink massive supplier since 2021 | ||||
| Quzhou Aifeimu Chemical Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (570) 604-0288 | |||
 |
info@afmpharm.com | |||
| Chemical manufacturer since 2011 | ||||
| chemBlink standard supplier since 2007 | ||||
| Nanjing Finetech Chemical Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (25) 5207-8417 +86 17714198479 | |||
 |
sales@fine-chemtech.com | |||
 |
QQ chat | |||
| Chemical manufacturer since 2007 | ||||
| chemBlink standard supplier since 2007 | ||||
| Fujian South Pharmaceutical Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (598) 286-0412 +86 13509335186 | |||
 |
sales@southpharma.com | |||
 |
QQ chat | |||
| Chemical manufacturer since 2001 | ||||
| chemBlink standard supplier since 2008 | ||||
| Institute of Petrochemistry, Heilongjiang Academy of Science | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (451) 8262-3696 +86 13945093324 | |||
 |
oledchem@gmail.com lkiwi2000@hotmail.com | |||
 |
QQ chat | |||
| Chemical manufacturer since 1962 | ||||
| chemBlink standard supplier since 2009 | ||||
| Heta Pharm & Chem Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (571) 8829-8691 | |||
 |
sales@hetapharm.com zhb0309@hotmail.com | |||
| Chemical manufacturer | ||||
| chemBlink standard supplier since 2009 | ||||
| Shanghai Yurlic Chemical S&T Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (21) 37285141 +86 15000957566 | |||
 |
yurlichem@hotmail.com sales@yurlichem.cn | |||
 |
QQ chat | |||
| Chemical manufacturer since 2004 | ||||
| chemBlink standard supplier since 2009 | ||||
| BOC Sciences | USA | Inquire | ||
|---|---|---|---|---|
 |
+1 (631) 485-4226 | |||
 |
info@bocsci.com | |||
| Chemical manufacturer | ||||
| chemBlink standard supplier since 2010 | ||||
| Classification | Biochemical >> Amino acids and their derivatives >> Amino alcohol derivative |
|---|---|
| Name | (R)-(+)-2-(Diphenylhydroxymethyl)pyrrolidine |
| Synonyms | alpha,alpha-Diphenyl-D-prolinol; (R)-(+)-alpha,alpha-Diphenyl-2-pyrrolidinemethanol |
| Molecular Structure | 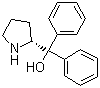 |
| Molecular Formula | C17H19NO |
| Molecular Weight | 253.34 |
| CAS Registry Number | 22348-32-9 |
| EC Number | 606-992-7 |
| SMILES | C1C[C@@H](NC1)C(C2=CC=CC=C2)(C3=CC=CC=C3)O |
| Density | 1.1±0.1 g/cm3 Calc.* |
|---|---|
| Melting point | 77 - 80 ºC (Expl.) |
| Boiling point | 411.3±12.0 ºC 760 mmHg (Calc.)* |
| Flash point | 133.3±10.1 ºC (Calc.)* |
| Index of refraction | 1.597 (Calc.)* |
| Alpha | 59 º (589 nm, c=3, meoh 25 ºC) (Expl.) |
| Hazard Symbols |
 GHS07 Warning Details
GHS07 Warning Details | ||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard Statements | H315-H319-H335 Details | ||||||||||||||||||||||||||||||||
| Precautionary Statements | P261-P264-P271-P280-P302+P352-P305+P351+P338 Details | ||||||||||||||||||||||||||||||||
| Hazard Classification | |||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||
| SDS | Available | ||||||||||||||||||||||||||||||||
|
(R)-(+)-2-(Diphenylhydroxymethyl)pyrrolidine is an organic compound that belongs to the class of chiral pyrrolidine derivatives. Its structure consists of a pyrrolidine ring substituted at the 2-position with a diphenylhydroxymethyl group. The presence of the stereogenic center at the carbon bearing the hydroxyl and diphenyl substituents gives rise to chirality, and the (R)-(+)-enantiomer is the optically active form that rotates plane-polarized light in the positive direction. The compound was first developed during research into chiral auxiliaries and ligands for asymmetric synthesis. In the late 20th century, chemists recognized the importance of small, rigid chiral molecules that could direct stereochemical outcomes in catalytic and stoichiometric transformations. (R)-(+)-2-(Diphenylhydroxymethyl)pyrrolidine emerged as one such compound, offering a combination of chirality, stability, and reactivity that made it attractive in stereoselective organic synthesis. Its primary application is as a chiral auxiliary and ligand in asymmetric synthesis. The hydroxyl group and the nitrogen atom in the pyrrolidine ring allow it to coordinate to metals or participate in hydrogen bonding, providing stereochemical control in a variety of transformations. It has been used in enantioselective reductions, nucleophilic additions, and catalytic reactions where high enantiomeric excess is required. In particular, (R)-(+)-2-(Diphenylhydroxymethyl)pyrrolidine is known as a component of the "Hayashi–Jørgensen catalyst," a family of organocatalysts used in asymmetric transformations such as aldol reactions and Michael additions. These catalysts exploit the chiral environment created by the pyrrolidine backbone and the bulky diphenyl group to control the stereochemistry of carbon–carbon bond formation, making them valuable tools in modern organic chemistry. Beyond catalysis, this compound is also studied as a building block for pharmaceuticals and fine chemicals. Its chirality and functional group arrangement make it a useful intermediate in the synthesis of bioactive molecules where enantiopure forms are critical for biological activity. Synthesis of (R)-(+)-2-(Diphenylhydroxymethyl)pyrrolidine typically involves enantioselective methods starting from chiral precursors or resolution of racemic mixtures. Its stability, crystalline nature, and ease of purification have contributed to its practical use in laboratories focused on asymmetric synthesis. Overall, (R)-(+)-2-(Diphenylhydroxymethyl)pyrrolidine represents an important chiral auxiliary and catalyst component in modern organic chemistry. Its discovery and application underscore the vital role of small, well-defined chiral molecules in enabling precise stereochemical control, which is essential for both research and industrial synthesis of enantiomerically pure compounds. References 2021. Development of a Chiral N-Alkoxyamide Strategy and Application to the Asymmetric Total Synthesis of Fasicularin. Synthesis, 53(18). DOI: 10.1055/a-1561-7815 2021. Enantioselective Functionalization of Prochiral Cyclobutanones and Cyclobutenones. Synlett, 32(14). DOI: 10.1055/a-1493-9489 1994. Methods for obtaining optically active intermediates for prostaglandin synthesis [review]. Pharmaceutical Chemistry Journal, 28(6). DOI: 10.1007/bf02285402 |
| Market Analysis Reports |
| List of Reports Available for (R)-(+)-2-(Diphenylhydroxymethyl)pyrrolidine |