Online Database of Chemicals from Around the World
| MolScanner | Singapore | Inquire | ||
|---|---|---|---|---|
 |
+86 18621675448 | |||
 |
marketing@molscanner.com | |||
 |
WhatsApp: 9896 7603 | |||
| Chemical manufacturer since 2025 | ||||
| chemBlink standard supplier since 2025 | ||||
| Classification | Pharmaceutical intermediate >> Heterocyclic compound intermediate >> Pyrimidine compound >> Carboxylic acid |
|---|---|
| Name | 2-Amino-4-bromo-3-fluoro-5-iodobenzoic acid |
| Molecular Structure | 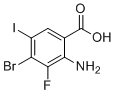 |
| Molecular Formula | C7H4BrFINO2 |
| Molecular Weight | 359.92 |
| CAS Registry Number | 2241720-33-0 |
| SMILES | C1=C(C(=C(C(=C1I)Br)F)N)C(=O)O |
| Density | 2.5±0.1 g/cm3 Calc.* |
|---|---|
| Boiling point | 392.9±42.0 ºC 760 mmHg (Calc.)* |
| Flash point | 191.4±27.9 ºC (Calc.)* |
| Index of refraction | 1.721 (Calc.)* |
| * | Calculated using Advanced Chemistry Development (ACD/Labs) Software. |
|
2-Amino-4-bromo-3-fluoro-5-iodobenzoic acid is a highly substituted aromatic carboxylic acid that belongs to the class of halogenated benzoic acid derivatives. It consists of a benzene ring bearing a carboxylic acid group, an amino group, and three halogen substituents: fluorine, bromine, and iodine. The positions of the substituents are specifically arranged as follows: the amino group at the 2-position, fluorine at the 3-position, bromine at the 4-position, iodine at the 5-position, and the carboxylic acid at the 1-position of the aromatic ring. This compound is structurally notable for the coexistence of three distinct halogen atoms on a single aromatic ring. Each halogen contributes uniquely to the reactivity and electronic character of the molecule. Fluorine, being highly electronegative and small, affects the electronic distribution of the aromatic system and often improves the metabolic stability and binding affinity of bioactive compounds. Bromine and iodine, larger and more polarizable atoms, serve as useful synthetic handles in cross-coupling reactions such as Suzuki, Sonogashira, or Buchwald–Hartwig couplings. These reactions enable the construction of more complex aromatic or heteroaromatic systems from this molecule as a versatile precursor. The amino group in the ortho-position relative to the carboxylic acid is a reactive nucleophilic site, and its placement allows for potential intramolecular hydrogen bonding. This structural feature can influence the molecule’s solubility and conformation, potentially affecting its interaction with biological targets. Additionally, the amino group is useful in the synthesis of amides, ureas, or other nitrogen-containing heterocycles, which are prevalent motifs in pharmaceuticals. Synthetically, 2-amino-4-bromo-3-fluoro-5-iodobenzoic acid can be prepared by stepwise halogenation of suitable aniline or benzoic acid derivatives. Each halogen is typically introduced under controlled conditions using halogenating agents such as N-bromosuccinimide for bromination, Selectfluor or N-fluorobenzenesulfonimide for fluorination, and iodine reagents like ICl for iodination. The directing effects of existing substituents guide the regioselectivity of each halogenation step. Due to its halogenation pattern and functional groups, this compound serves as a valuable intermediate in medicinal chemistry and materials science. It allows for further derivatization toward small-molecule libraries used in drug discovery or as building blocks for radiolabeled imaging agents. Its capacity for selective functionalization at different positions makes it especially suitable for structure-activity relationship studies, where systematic modifications are required to fine-tune biological activity or physicochemical properties. In summary, 2-amino-4-bromo-3-fluoro-5-iodobenzoic acid is a densely functionalized benzoic acid derivative with applications in synthetic chemistry. Its distinctive combination of an amino group and three differently reactive halogens provides multiple pathways for chemical transformation, supporting its use as a key intermediate in the development of advanced molecules for pharmaceutical or diagnostic purposes. |
| Market Analysis Reports |
| List of Reports Available for 2-Amino-4-bromo-3-fluoro-5-iodobenzoic acid |