Online Database of Chemicals from Around the World
| Jiangsu Nengyue New Material Technology Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 13376001577 | |||
 |
robin@jiangsunengyue.com | |||
 |
WhatsApp: +8613376001577 | |||
| Chemical manufacturer since 2022 | ||||
| chemBlink standard supplier since 2024 | ||||
| Classification | Organic raw materials >> Nitrile compound |
|---|---|
| Name | 2-(4-methoxyphenyl)-2-(5-((tosyloxy)imino)thiophen-2(5H)-ylidene)acetonitrile |
| Synonyms | (5-p-Toluene Sulfony Oxygen Imide-5H-thiophene-2-ylidene)-(4-Methoxyphenyl)acetonitrile |
| Molecular Structure | 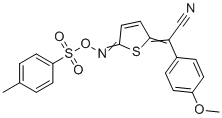 |
| Molecular Formula | C20H16N2O4S2 |
| Molecular Weight | 412.48 |
| CAS Registry Number | 2270831-00-8 |
| SMILES | C1=CC(=CC=C1[S](=O)(=O)ON=C2C=CC(S2)=C(C#N)C3=CC=C(C=C3)OC)C |
|
The chemical compound 2-(4-methoxyphenyl)-2-(5-((tosyloxy)imino)thiophen-2(5H)-ylidene)acetonitrile represents an interesting development in organic synthesis, particularly within the realm of heterocyclic compounds. Its discovery is closely linked to the advancements in thiophene chemistry, a class of sulfur-containing heterocyclic compounds known for their unique electronic properties and versatile applications. Thiophene-based compounds have attracted significant attention due to their role in materials science, organic electronics, and medicinal chemistry. The incorporation of a nitrile group and a tosyloxy-imino functional group in this compound further enhances its chemical reactivity and potential for various synthetic transformations. The compound was first synthesized as part of efforts to create new materials with enhanced electronic properties. The thiophene core, combined with the electron-withdrawing nitrile and electron-donating methoxyphenyl groups, results in a structure with a highly conjugated system. This conjugation plays a crucial role in its application in organic electronics, particularly in the development of organic semiconductors and photovoltaic materials. The presence of the tosyloxy-imino group adds functionality, making it a useful intermediate in coupling reactions and other organic transformations. In synthetic organic chemistry, 2-(4-methoxyphenyl)-2-(5-((tosyloxy)imino)thiophen-2(5H)-ylidene)acetonitrile is used as a building block for the preparation of more complex molecules. Its reactivity allows for the formation of various heterocyclic frameworks, which are important in the development of pharmaceuticals and agrochemicals. The imino functionality offers a platform for further derivatization, making the compound valuable in multistep synthesis strategies. Additionally, the nitrile group provides a handle for reactions such as nucleophilic addition and substitution, broadening its application in synthetic methodologies. This compound has also been explored for its potential in medicinal chemistry, particularly as a scaffold for drug discovery. Thiophene derivatives are known for their biological activities, including anti-inflammatory, antiviral, and anticancer properties. The specific structural features of this compound, such as the methoxyphenyl group and the imino functionality, may contribute to its interaction with biological targets, though further research is needed to fully explore its therapeutic potential. Another key application of 2-(4-methoxyphenyl)-2-(5-((tosyloxy)imino)thiophen-2(5H)-ylidene)acetonitrile is in materials science, where it can be used in the development of novel polymers and advanced materials. Its electron-rich thiophene core, combined with the functional groups, makes it suitable for creating conductive polymers and other materials used in sensors, displays, and energy storage devices. The compound's ability to participate in polymerization reactions, while maintaining its structural integrity, is crucial for these applications. The synthesis of this compound typically involves the condensation of a methoxyphenyl acetonitrile derivative with a thiophene-based intermediate, followed by the introduction of the tosyloxy-imino group. This multi-step process highlights the versatility of the starting materials and the importance of careful reaction optimization to achieve the desired product. Given its reactivity and functionality, the compound is usually handled in controlled laboratory environments, with attention to proper safety measures due to the presence of potentially reactive groups. |
| Market Analysis Reports |
| List of Reports Available for 2-(4-methoxyphenyl)-2-(5-((tosyloxy)imino)thiophen-2(5H)-ylidene)acetonitrile |