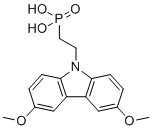
(2-(3,6-Dimethoxy-9H-carbazol-9-yl)ethyl)phosphonic acid is an organic compound that has been studied for its unique chemical structure and potential applications in various fields, particularly in electronics and materials science. The molecule consists of a carbazole backbone, a heterocyclic aromatic compound, with two methoxy groups at the 3 and 6 positions, and a phosphonic acid group attached to an ethyl chain. The presence of the phosphonic acid functional group and the carbazole structure gives this compound unique properties, making it useful in various applications, such as organic electronics and as a building block in molecular electronics.
The discovery of (2-(3,6-Dimethoxy-9H-carbazol-9-yl)ethyl)phosphonic acid emerged from the need for novel materials in organic electronics, especially those with good charge-transporting properties and the ability to form stable interfaces with other materials. Carbazole derivatives, in particular, have been widely studied for their excellent electronic properties, including high charge carrier mobility, fluorescence, and good stability. The addition of the phosphonic acid group was aimed at enhancing the solubility and processability of the material, while also allowing for strong interactions with metal oxide surfaces, which is critical in the fabrication of organic electronic devices.
One of the major applications of (2-(3,6-Dimethoxy-9H-carbazol-9-yl)ethyl)phosphonic acid is in the field of organic light-emitting diodes (OLEDs). OLEDs are used in displays and lighting applications due to their flexibility, energy efficiency, and ease of manufacturing. Carbazole derivatives are frequently employed as the electron transport materials in OLED devices because of their favorable electronic properties. The phosphonic acid group in (2-(3,6-Dimethoxy-9H-carbazol-9-yl)ethyl)phosphonic acid enhances the material's ability to form stable interfaces with metal oxide layers, which is a critical factor in improving the device's efficiency and stability. Additionally, the methoxy groups on the carbazole ring contribute to the solubility of the compound, making it easier to incorporate into thin film coatings.
Another important application is in organic photovoltaics (OPVs), where (2-(3,6-Dimethoxy-9H-carbazol-9-yl)ethyl)phosphonic acid can be used as a donor material or as part of the active layer. Organic photovoltaics are an emerging technology that promises to offer low-cost, flexible, and lightweight solar cells. The phosphonic acid group in this compound allows it to form strong interactions with metal oxide electrodes, improving the performance and stability of the devices. Furthermore, the carbazole unit, known for its good hole-transport properties, helps to facilitate the movement of charge carriers within the device.
The compound has also been studied for its potential in molecular electronics, where it could serve as a building block for self-assembled monolayers (SAMs) and other nanoscale devices. The ability of the phosphonic acid group to form strong bonds with metal surfaces makes it a promising candidate for such applications. In these systems, the molecule's ability to organize itself into structured monolayers could be leveraged for creating novel nanoscale electronic components.
The synthesis of (2-(3,6-Dimethoxy-9H-carbazol-9-yl)ethyl)phosphonic acid typically involves the functionalization of carbazole derivatives with ethyl phosphonic acid groups. Various methods have been employed to achieve the desired functionalization, including nucleophilic substitution reactions and coupling reactions. The overall process is designed to preserve the electronic properties of the carbazole unit while incorporating the phosphonic acid functionality, which enhances the material's interaction with metal oxide surfaces and its processability in electronic devices.
In conclusion, (2-(3,6-Dimethoxy-9H-carbazol-9-yl)ethyl)phosphonic acid is a promising organic compound with a range of potential applications in organic electronics, including OLEDs, OPVs, and molecular electronics. Its unique structure, which combines the electronic properties of carbazole with the functionality of phosphonic acid, positions it as a valuable material for future developments in flexible, low-cost, and high-performance electronic devices.
|


 GHS07 Warning Details
GHS07 Warning Details