3-Amino-4-pyrazolecarboxamide hemisulfate is a chemical compound consisting of the pyrazolecarboxamide core functionalized with an amino group at the 3-position of the pyrazole ring. The compound exists as a hemisulfate salt, meaning it is associated with half an equivalent of sulfuric acid, which typically forms during salt preparation to enhance solubility and stability.
The pyrazolecarboxamide moiety is a heterocyclic structure featuring a five-membered pyrazole ring fused with a carboxamide group at the 4-position. The introduction of an amino group at the 3-position provides additional hydrogen bonding capability and modifies the electronic character of the ring. Pyrazole derivatives have attracted considerable interest due to their broad pharmacological properties, including antimicrobial, anti-inflammatory, and anticancer activities.
The hemisulfate salt form of 3-amino-4-pyrazolecarboxamide improves the compound’s physicochemical properties, especially solubility in aqueous media, which is crucial for formulation and biological evaluation. The salt formation is commonly achieved by treatment of the free base compound with sulfuric acid under controlled conditions, resulting in protonation of nitrogen atoms in the pyrazole ring or the amino group.
Synthesis of 3-amino-4-pyrazolecarboxamide involves the construction of the pyrazole ring, typically via cyclization of suitable hydrazine and β-ketoamide precursors, followed by functional group modifications to introduce the amino substituent. The carboxamide group is introduced through amide formation reactions on corresponding pyrazole carboxylic acid derivatives or by direct substitution during ring synthesis.
Chemically, the compound exhibits typical behavior of pyrazolecarboxamides, including the ability to participate in hydrogen bonding, tautomeric equilibria, and nucleophilic or electrophilic substitution reactions on the pyrazole ring. The amino substituent enhances basicity and may serve as a reactive site for further chemical derivatization or conjugation.
In medicinal chemistry, 3-amino-4-pyrazolecarboxamide and its derivatives have been investigated for their potential biological activities. The pyrazole scaffold is a common motif in various drugs and drug candidates due to its ability to interact with diverse biological targets such as enzymes, receptors, and ion channels. The presence of both amino and carboxamide functionalities increases the likelihood of forming strong interactions with protein active sites.
Analytical characterization includes nuclear magnetic resonance (²H and ³C NMR) spectroscopy to confirm ring substitution patterns and the presence of amino and amide protons. Infrared (IR) spectroscopy identifies characteristic amide carbonyl stretching near 1650 cm−1 and N–H stretches from both amino and amide groups. Mass spectrometry verifies molecular weight and fragmentation consistent with the pyrazolecarboxamide structure.
Physically, the hemisulfate salt is typically a crystalline solid with enhanced aqueous solubility compared to the free base, facilitating its use in biological assays and pharmaceutical formulations. The salt is generally stable under standard storage conditions.
In summary, 3-amino-4-pyrazolecarboxamide hemisulfate is a heterocyclic compound combining a pyrazole ring substituted with amino and carboxamide groups and stabilized as a hemisulfate salt. Its chemical features and salt form confer properties favorable for use as an intermediate or active compound in pharmaceutical research and synthetic chemistry.
|


















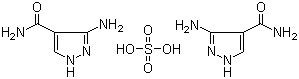

 GHS07;GHS09 Warning Details
GHS07;GHS09 Warning Details