Online Database of Chemicals from Around the World
| Capot Chemical Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (571) 8558-6718 +86 13336195806 | |||
 |
capotchem@gmail.com sales@capotchem.com | |||
 |
QQ chat | |||
| Chemical manufacturer | ||||
| chemBlink standard supplier since 2006 | ||||
| Xinghua Mingwei Chemical Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (523) 8374-8465 8374-8464 | |||
 |
1344539341@qq.com tmm@msbiochem.com | |||
| Chemical manufacturer | ||||
| chemBlink standard supplier since 2007 | ||||
| Jintan Hunter Chemical Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (519) 8280-8688 8218-0068 | |||
 |
admin@hunterchem.com.cn | |||
| Chemical manufacturer since 2008 | ||||
| chemBlink standard supplier since 2007 | ||||
| Jiangsu Haixiang Chemical Industry Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (519) 8019-1677 | |||
 |
michaelliorama@gmail.com | |||
| Chemical manufacturer | ||||
| chemBlink standard supplier since 2007 | ||||
| Simagchem Corporation | China | Inquire | ||
|---|---|---|---|---|
 |
+86 13806087780 | |||
 |
sale@simagchem.com | |||
| Chemical manufacturer since 2002 | ||||
| chemBlink standard supplier since 2008 | ||||
| Yizheng East Chemical Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (514) 8796-1069 | |||
 |
fzj@chinaeastchem.com fang_yizhengeast@hotmail.com | |||
| Chemical manufacturer since 1989 | ||||
| chemBlink standard supplier since 2008 | ||||
| Dagro Chemical (Changzhou) Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (519) 8513-8680 | |||
 |
daiyu@dagrochem.com daiyu0917@hotmail.com | |||
 |
QQ chat | |||
| Chemical manufacturer since 2005 | ||||
| chemBlink standard supplier since 2009 | ||||
| Extrasynthese Chemical S.A.S. | France | Inquire | ||
|---|---|---|---|---|
 |
+33 (47) 898-2034 | |||
 |
info@extrasynthese.com | |||
| Chemical manufacturer | ||||
| chemBlink standard supplier since 2009 | ||||
| Classification | Chemical reagent >> Organic reagent >> Aromatic acid |
|---|---|
| Name | 2,6-Dihydroxybenzoic acid |
| Synonyms | 2,6-Resorcylic acid; gamma-Resorcylic acid |
| Molecular Structure | 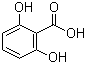 |
| Molecular Formula | C7H6O4 |
| Molecular Weight | 154.12 |
| CAS Registry Number | 303-07-1 |
| EC Number | 206-134-8 |
| SMILES | C1=CC(=C(C(=C1)O)C(=O)O)O |
| Density | 1.6±0.1 g/cm3, Calc.* |
|---|---|
| Melting point | 165 ºC (decomp.) |
| Index of Refraction | 1.671, Calc.* |
| Boiling Point | 343.7±32.0 ºC (760 mmHg), Calc.* |
| Flash Point | 175.8±21.6 ºC, Calc.* |
| * | Calculated using Advanced Chemistry Development (ACD/Labs) Software. |
| Hazard Symbols |
 GHS07 Warning Details
GHS07 Warning Details | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard Statements | H315-H319-H335 Details | ||||||||||||||||||||
| Precautionary Statements | P261-P264-P264+P265-P271-P280-P302+P352-P304+P340-P305+P351+P338-P319-P321-P332+P317-P337+P317-P362+P364-P403+P233-P405-P501 Details | ||||||||||||||||||||
| Hazard Classification | |||||||||||||||||||||
| |||||||||||||||||||||
| SDS | Available | ||||||||||||||||||||
|
2,6-Dihydroxybenzoic acid, also known as salicylic acid or 2,6-dihydroxybenzenecarboxylic acid, is an organic compound with the molecular formula C7H6O4. This compound is a derivative of benzoic acid, characterized by the presence of two hydroxyl groups located at the 2 and 6 positions of the aromatic ring. The discovery of 2,6-dihydroxybenzoic acid dates back to the late 19th century when it was first isolated from the bark of willow trees (Salix species). Its historical significance is attributed to its association with the analgesic and anti-inflammatory properties of salicylates, which led to the development of aspirin (acetylsalicylic acid). The applications of 2,6-dihydroxybenzoic acid are extensive, particularly in the fields of medicine, agriculture, and analytical chemistry. One of its most notable uses is in the pharmaceutical industry, where it serves as a precursor for the synthesis of various salicylate derivatives. These compounds are well-known for their anti-inflammatory, analgesic, and antipyretic properties. Salicylic acid itself is commonly used in the formulation of topical treatments for acne and psoriasis, as it helps to exfoliate the skin and reduce inflammation. In agriculture, 2,6-dihydroxybenzoic acid has been identified as a plant growth regulator. It plays a role in enhancing plant resistance to environmental stress, such as drought and disease. Its application as a biostimulant promotes root development and overall plant vigor, making it valuable in sustainable agriculture practices. Researchers have explored its potential in improving crop yields and enhancing the nutritional quality of various food crops. Moreover, 2,6-dihydroxybenzoic acid is utilized in analytical chemistry, particularly in the development of various analytical methods for detecting metal ions. It can form stable complexes with certain metal ions, facilitating their determination in environmental samples. The compound has been employed in spectrophotometric methods for the analysis of heavy metals, contributing to environmental monitoring efforts. The synthesis of 2,6-dihydroxybenzoic acid typically involves the hydroxylation of benzoic acid or the oxidation of 2,6-dihydroxybenzaldehyde. These synthetic routes are straightforward and allow for the efficient production of the compound for research and industrial applications. In summary, 2,6-dihydroxybenzoic acid is a compound of significant interest due to its diverse applications in medicine, agriculture, and analytical chemistry. Its historical importance, stemming from its association with salicylates and their therapeutic effects, continues to be relevant today. Ongoing research into its properties and potential uses may lead to further advancements in various fields, particularly in the development of sustainable agricultural practices and novel therapeutic agents. References 2024. A pushed biosynthesis of 2,6-dihydroxybenzoic acid by the recombinant 2,3-dihydroxybenzoic acid decarboxylase immobilized on novel amino-modified lignin-containing cellulose nanocrystal aerogel. Bioresource Technology, 393. DOI: 10.1016/j.biortech.2023.130218 2022. Study on the interaction between 2,6-dihydroxybenzoic acid nicotine salt and human serum albumin by multi-spectroscopy and molecular dynamics simulation. Spectrochimica Acta. Part A, Molecular and Biomolecular Spectroscopy, 270. DOI: 10.1016/j.saa.2022.120868 2023. Chemical characterization, in vitro antioxidant, anti-cancer and enzyme inhibition activities of three edible mushroom species. Journal of Food Measurement and Characterization, 17(5). DOI: 10.1007/s11694-023-02066-5 |
| Market Analysis Reports |
| List of Reports Available for 2,6-Dihydroxybenzoic acid |