Online Database of Chemicals from Around the World
| Shanghai Worldyang Chemical Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 13651600618 +86 (21) 5679-5779 | |||
 |
sales7777@worldyachem.com | |||
 |
QQ chat | |||
 |
WeChat: 13651600618 | |||
 |
WhatsApp: +86 13651600618 | |||
| Chemical manufacturer since 2012 | ||||
| chemBlink premium supplier since 2023 | ||||
| Classification | Organic raw materials >> Organometallic compound >> Organic rhodium |
|---|---|
| Name | Bis(1,5-cyclooctadiene)rhodium(I) tetrafluoroborate |
| Synonyms | (1Z,5Z)-cycloocta-1,5-diene;rhodium tetrafluoroborate |
| Molecular Structure | 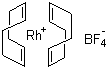 |
| Molecular Formula | C16H24Rh.BF4 |
| Molecular Weight | 406.07 |
| CAS Registry Number | 35138-22-8 |
| EC Number | 609-074-4 |
| SMILES | [B-](F)(F)(F)F.C1/C=C\CC/C=C\C1.C1/C=C\CC/C=C\C1.[Rh] |
| Melting point | ~190 ºC (dec.) (Expl.) |
|---|---|
| Water solubility | insoluble |
| Hazard Symbols |

 GHS07;GHS08 Danger Details
GHS07;GHS08 Danger Details | ||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard Statements | H302-H315-H317-H319-H334-H335 Details | ||||||||||||||||||||||||||||||||||||||||||||
| Precautionary Statements | P233-P260-P261-P264-P264+P265-P270-P271-P272-P280-P284-P301+P317-P302+P352-P304+P340-P305+P351+P338-P319-P321-P330-P332+P317-P333+P317-P337+P317-P342+P316-P362+P364-P403-P403+P233-P405-P501 Details | ||||||||||||||||||||||||||||||||||||||||||||
| Hazard Classification | |||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||
| Transport Information | UN 2921 | ||||||||||||||||||||||||||||||||||||||||||||
| SDS | Available | ||||||||||||||||||||||||||||||||||||||||||||
|
Bis(1,5-cyclooctadiene)rhodium(I) tetrafluoroborate is a coordination complex consisting of a rhodium(I) metal center coordinated to two 1,5-cyclooctadiene (COD) ligands, with a tetrafluoroborate (BF4-) counterion balancing the charge. The complex is commonly denoted as \[Rh(COD)2]+ BF4- and features rhodium in the +1 oxidation state. Structurally, the rhodium ion is coordinated in a square planar geometry by the two COD ligands, which each bind through their conjugated diene systems via π-bonding. The coordination of COD ligands stabilizes the low oxidation state of rhodium and facilitates the complex’s reactivity as a precursor in homogeneous catalysis. The complex is synthesized by the reaction of rhodium(I) chloride dimers with silver tetrafluoroborate in the presence of excess 1,5-cyclooctadiene. The silver salt abstracts chloride ions, forming insoluble silver chloride and yielding the soluble cationic rhodium complex paired with tetrafluoroborate anions. Bis(1,5-cyclooctadiene)rhodium(I) tetrafluoroborate is widely used as a catalyst precursor or catalyst component in various organic transformations. Its role is significant in facilitating reactions such as hydrogenation, hydroformylation, carbonylation, and carbon–carbon coupling. The complex’s relatively labile COD ligands can be displaced by substrates or other ligands, activating the rhodium center for catalytic cycles. Its advantages include good solubility in common organic solvents, ease of handling compared to neutral rhodium complexes, and the ability to generate active catalytic species under mild conditions. The tetrafluoroborate counterion is non-coordinating, which helps maintain the complex's cationic nature and catalytic activity. In synthetic chemistry, this complex is valued for generating rhodium catalysts with tunable activity and selectivity by ligand substitution. It serves as a versatile starting material for preparing rhodium complexes bearing phosphines, N-heterocyclic carbenes, or other ligands, tailored for specific catalytic applications. Physicochemical properties include being a red-orange solid with stability under inert atmosphere but sensitivity to moisture and air, which can lead to oxidation or ligand exchange. The complex is soluble in solvents such as dichloromethane, tetrahydrofuran, and toluene. Analytical methods such as nuclear magnetic resonance (NMR) spectroscopy, infrared (IR) spectroscopy, and elemental analysis are used to confirm the structure and purity of the complex. The COD ligands display characteristic signals in NMR spectra, while IR can detect metal-ligand vibrations. In summary, bis(1,5-cyclooctadiene)rhodium(I) tetrafluoroborate is a key rhodium(I) coordination complex widely employed as a catalyst precursor in homogeneous catalysis. Its combination of labile COD ligands and a non-coordinating tetrafluoroborate counterion provides a reactive yet stable platform for diverse catalytic transformations in organic synthesis. References 2024. Rh-catalysed enantioselective [2+2+1] cycloaddition reactions using three different 2π-components. Nature Synthesis, 3(7). DOI: 10.1038/s44160-024-00604-7 2022. Stereoselective cyclohexadienylamine synthesis through rhodium-catalysed [2+2+2] cyclotrimerization. Nature Synthesis, 1(3). DOI: 10.1038/s44160-022-00043-2 2016. Merging rhodium-catalysed C-H activation and hydroamination in a highly selective [4+2] imine/alkyne annulation. Nature Communications, 7. DOI: 10.1038/ncomms11506 |
| Market Analysis Reports |
| List of Reports Available for Bis(1,5-cyclooctadiene)rhodium(I) tetrafluoroborate |