Online Database of Chemicals from Around the World
| Hefei TNJ Chemical Industry Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (551) 6541-8684 | |||
 |
sales@tnjchem.com | |||
| Chemical manufacturer since 2001 | ||||
| chemBlink standard supplier since 2010 | ||||
| Yangzhou Model Electronics Materials Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 13761402923 | |||
 |
245662540@qq.com | |||
| Chemical manufacturer since 2018 | ||||
| chemBlink standard supplier since 2021 | ||||
| P and M Invest Ltd. | Russia | Inquire | ||
|---|---|---|---|---|
 |
+7 (495) 135-6494 | |||
 |
sales@fluorine1.ru | |||
| Chemical manufacturer | ||||
| Classification | Chemical reagent >> Organic reagent >> Oxime |
|---|---|
| Name | Trifluoroacetamidine |
| Molecular Structure | 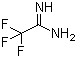 |
| Molecular Formula | C2H3F3N2 |
| Molecular Weight | 112.05 |
| CAS Registry Number | 354-37-0 |
| EC Number | 670-531-6 |
| SMILES | C(=N)(C(F)(F)F)N |
| Density | 1.6±0.1 g/cm3 Calc.*, 1.494 g/mL (Expl.) |
|---|---|
| Boiling point | 58.2±40.0 ºC 760 mmHg (Calc.)*, 159.6 - 162.4 ºC (Expl.) |
| Flash point | -11.0±27.3 ºC (Calc.)* |
| Index of refraction | 1.357 (Calc.)*, 1.382 (Expl.) |
| * | Calculated using Advanced Chemistry Development (ACD/Labs) Software. |
| Hazard Symbols |

 GHS05;GHS07 Danger Details
GHS05;GHS07 Danger Details | ||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard Statements | H302+H312+H332-H302-H312-H314-H332 Details | ||||||||||||||||||||||||||||||||
| Precautionary Statements | P260-P261-P264-P270-P271-P280-P301+P317-P301+P330+P331-P302+P352-P302+P361+P354-P304+P340-P305+P354+P338-P316-P317-P321-P330-P362+P364-P363-P405-P501 Details | ||||||||||||||||||||||||||||||||
| Hazard Classification | |||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||
| SDS | Available | ||||||||||||||||||||||||||||||||
|
Trifluoroacetamidine is a fluorinated amidine compound structurally characterized by the presence of a trifluoromethyl group (–CF3) attached to the carbon atom of the amidine functional group. Its chemical formula is CF3C(=NH)NH2, and it can exist in various protonation or tautomeric forms depending on the medium. Trifluoroacetamidine has attracted attention due to the strong electron-withdrawing effect of the trifluoromethyl group, which influences the basicity and nucleophilicity of the nitrogen atoms within the amidine moiety. The discovery and synthesis of trifluoroacetamidine are closely linked to the broader development of trifluoromethyl-containing organic compounds in the mid-20th century. With growing interest in fluorinated molecules for pharmaceuticals, agrochemicals, and specialty materials, chemists began to explore methods for introducing the CF3 group into various functional frameworks. Trifluoroacetamidine was synthesized through methods involving nucleophilic substitution on trifluoroacetonitrile followed by treatment with ammonia or amines, leading to amidine formation. Such syntheses were important steps in understanding the behavior of CF3-substituted electrophiles and nucleophiles. In terms of application, trifluoroacetamidine serves as a valuable synthetic intermediate, especially in the preparation of heterocyclic compounds and fluorinated ligands. One of its most notable uses is in the synthesis of trifluoroacetamidines as ligands or intermediates for metal complexation reactions. The presence of the electron-withdrawing CF3 group significantly modulates the electronic properties of the amidine, making it a suitable donor ligand in coordination chemistry. It can coordinate to metal centers through one or both nitrogen atoms, often forming stable chelates useful in catalysis or materials science. Another important application of trifluoroacetamidine is its use in peptide and protein chemistry. In particular, its derivatives can act as enzyme inhibitors or transition-state analogs. The CF3 group enhances metabolic stability and bioavailability, characteristics often sought in drug design. Additionally, trifluoroacetamidine derivatives have been explored in the development of fluorinated pharmaceuticals, where they may serve as precursors or active motifs influencing binding affinities and pharmacokinetics. In organic synthesis, trifluoroacetamidine is also employed as a precursor to other trifluoromethylated compounds. For example, it can be transformed into trifluoroacetimidoyl chlorides or trifluoroacetylated products, which are useful intermediates in constructing more complex molecular architectures. Because of the stability and versatility of the CF3 group, such intermediates find use in agrochemical and medicinal compound development, especially where increased lipophilicity or metabolic resistance is desired. From a chemical reactivity standpoint, the presence of the CF3 group adjacent to the amidine moiety strongly reduces the basicity of the nitrogen atoms compared to unsubstituted amidines. This has been confirmed by pKa measurements and spectroscopic studies. As a result, trifluoroacetamidine and its derivatives often require activation or specific reaction conditions to undergo nucleophilic or electrophilic transformations. Trifluoroacetamidine is generally a crystalline or hygroscopic solid, soluble in polar organic solvents such as methanol, acetonitrile, or dimethylformamide. Like many nitrogen-containing small molecules, it should be handled with care due to its potential for skin and respiratory irritation. Appropriate personal protective equipment and fume hood usage are recommended during handling. Its chemical stability under ambient conditions makes it a convenient reagent in synthetic applications. Although it is not a high-volume industrial chemical, trifluoroacetamidine remains relevant in specialized research areas and fine chemical synthesis. Its role as a building block in the preparation of biologically active fluorinated compounds, ligands for metal complexes, and intermediates in organofluorine chemistry ensures its continued utility in both academic and applied chemical research. References 1987 Synthesis of 2-trifluoromethyl-4,6-diphenyl-1,3,5-triazine. Bulletin of the Academy of Sciences of the USSR, Division of chemical science, 35(4). DOI: 10.1007/bf00954241 2014 2-Trifluoromethyl-1,3-diazabutadienes as Useful Intermediates for the Construction of 2-Trifluoromethylpyrimidine Derivatives. Synthesis, 50(15). DOI: 10.1055/s-0037-1610444 2014 Fluorine Containing Diazines. Synthesis and Properties. Fluorine in Heterocyclic Chemistry Volume 2. DOI: 10.1007/978-3-319-04435-4_6 |
| Market Analysis Reports |
| List of Reports Available for Trifluoroacetamidine |