Online Database of Chemicals from Around the World
| Spec-Chem Industry Inc. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (25) 8452-3390 ex 111 | |||
 |
sc@specchemind.com | |||
| Chemical manufacturer since 1995 | ||||
| chemBlink standard supplier since 2006 | ||||
| Simagchem Corporation | China | Inquire | ||
|---|---|---|---|---|
 |
+86 13806087780 | |||
 |
sale@simagchem.com | |||
| Chemical manufacturer since 2002 | ||||
| chemBlink standard supplier since 2008 | ||||
| BOC Sciences | USA | Inquire | ||
|---|---|---|---|---|
 |
+1 (631) 485-4226 | |||
 |
info@bocsci.com | |||
| Chemical manufacturer | ||||
| chemBlink standard supplier since 2010 | ||||
| Creative Peptides | USA | Inquire | ||
|---|---|---|---|---|
 |
+1 (631) 624-4882 | |||
 |
info@creative-peptides.com | |||
| Chemical manufacturer | ||||
| chemBlink standard supplier since 2010 | ||||
| Biosynth AG. | Switzerland | Inquire | ||
|---|---|---|---|---|
 |
+41 (71) 858-2020 | |||
 |
welcome@biosynth.ch | |||
| Chemical manufacturer | ||||
| chemBlink standard supplier since 2014 | ||||
| Labseeker Inc | USA | Inquire | ||
|---|---|---|---|---|
 |
+1 (858) 750-1632 | |||
 |
marketing@labseeker.com | |||
| Chemical manufacturer since 2002 | ||||
| chemBlink standard supplier since 2015 | ||||
| Hangzhou Leap Chem Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (571) 8771-1850 | |||
 |
market19@leapchem.com | |||
 |
QQ chat | |||
| Chemical manufacturer since 2006 | ||||
| chemBlink standard supplier since 2015 | ||||
| Changzhou Jiuwu Chemical Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (519) 8512-1086 +86 13775162768 | |||
 |
info@jiuwuchem.cn | |||
 |
QQ chat | |||
| Chemical manufacturer since 2009 | ||||
| chemBlink standard supplier since 2023 | ||||
| Classification | Organic raw materials >> Heterocyclic compound >> Indoles |
|---|---|
| Name | Ethyl indole-2-carboxylate |
| Synonyms | Indole-2-carboxylic acid ethyl ester |
| Molecular Structure | 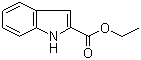 |
| Molecular Formula | C11H11NO2 |
| Molecular Weight | 189.21 |
| CAS Registry Number | 3770-50-1 |
| EC Number | 223-206-4 |
| SMILES | CCOC(=O)C1=CC2=CC=CC=C2N1 |
| Density | 1.2±0.1 g/cm3, Calc.* |
|---|---|
| Melting point | 122-125 ºC (Expl.) |
| Index of Refraction | 1.621, Calc.* |
| Boiling Point | 342.4±15.0 ºC (760 mmHg), Calc.* |
| Flash Point | 160.9±20.4 ºC, Calc.* |
| * | Calculated using Advanced Chemistry Development (ACD/Labs) Software. |
| Hazard Symbols |
 GHS07 Warning Details
GHS07 Warning Details | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard Statements | H315-H319 Details | ||||||||||||||||
| Precautionary Statements | P264-P264+P265-P280-P302+P352-P305+P351+P338-P321-P332+P317-P337+P317-P362+P364 Details | ||||||||||||||||
| Hazard Classification | |||||||||||||||||
| |||||||||||||||||
| SDS | Available | ||||||||||||||||
|
Ethyl indole-2-carboxylate is an organic compound that belongs to the indole class of heterocyclic aromatic compounds. It has the chemical formula C9H9NO2 and consists of an indole ring structure with a carboxylate ester group (ethyl ester) attached at the 2-position of the ring. Indoles and their derivatives are significant in various areas of chemistry due to their diverse biological activities and chemical reactivity. Ethyl indole-2-carboxylate is notable for its role as an intermediate in the synthesis of bioactive molecules, particularly in pharmaceutical and agricultural chemistry. The discovery of ethyl indole-2-carboxylate is tied to the growing interest in indole derivatives, which have been known for their presence in a wide variety of natural products, such as alkaloids and hormones. Indoles are also found in many important biological systems, including neurotransmitters like serotonin. The synthetic routes to ethyl indole-2-carboxylate typically involve standard methods for esterification of indole-2-carboxylic acid, such as reaction with ethanol in the presence of an acid catalyst. The development of its synthetic methodology has enabled researchers to explore its reactivity and potential applications. Ethyl indole-2-carboxylate has proven to be a valuable intermediate in organic synthesis, especially in the production of pharmaceuticals. It is often used in the synthesis of more complex indole-based molecules, which are of significant interest due to their broad spectrum of pharmacological activities, such as anti-inflammatory, anticancer, and antimicrobial properties. Indole derivatives, including ethyl indole-2-carboxylate, have been studied for their potential as kinase inhibitors, which could be beneficial in cancer therapies, and as modulators of central nervous system functions, especially in the treatment of neurological diseases. In addition to its applications in medicinal chemistry, ethyl indole-2-carboxylate is utilized in the synthesis of agrochemicals, particularly pesticides. Indole derivatives have been identified as useful scaffolds in the development of herbicides and fungicides, and ethyl indole-2-carboxylate is no exception. The compound’s ability to undergo a variety of chemical reactions, such as nucleophilic substitution and Friedel-Crafts reactions, enables it to serve as a precursor to more complex agrochemical products. These applications are important in crop protection, where efficient and selective chemical agents are required to control pests and weeds. The chemical versatility of ethyl indole-2-carboxylate also extends to materials science. The compound can undergo various transformations, such as cyclization reactions and cross-coupling, leading to the synthesis of novel organic compounds with applications in optoelectronic devices, sensors, and polymers. The presence of the indole ring structure imparts certain electronic properties to the molecule, which is of interest for the development of new materials with specialized functionalities. Furthermore, ethyl indole-2-carboxylate is also being investigated for its role in the synthesis of bioactive natural products and its use in the design of new drug candidates. The compound’s ability to act as a building block for a wide range of bioactive indole derivatives makes it a valuable tool in the development of new treatments for diseases ranging from cancer to infectious diseases. In conclusion, ethyl indole-2-carboxylate is an important indole derivative that has broad applications in synthetic chemistry, medicinal chemistry, agrochemicals, and materials science. Its role as a versatile intermediate in the synthesis of bioactive molecules has made it a valuable compound in the development of new therapeutic agents and functional materials. Ongoing research into its derivatives continues to explore new and innovative uses for this compound, particularly in the fields of drug discovery and advanced materials. References 2023. Synthesis of indole-based ferulic acid derivatives and in vitro evaluation of antiviral activity against SARS-CoV-2. Medicinal Chemistry Research, 32(11). DOI: 10.1007/s00044-023-03134-7 2020. Synthetic strategies, SAR studies, and computer modeling of indole 2 and 3-carboxamides as the strong enzyme inhibitors: a review. Molecular Diversity, 24(4). DOI: 10.1007/s11030-020-10061-x 2017. Copper(I)-Catalyzed Enantioselective Borylative Dearomatization of Indoles. Synthesis of Functionalized Organoboron Compounds Through Copper(I) Catalysis [No volume/issue available]. DOI: 10.1007/978-981-10-4935-4_7 |
| Market Analysis Reports |
| List of Reports Available for Ethyl indole-2-carboxylate |