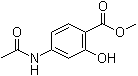
Methyl 4-(acetylamino)-2-hydroxybenzoate is an aromatic ester that belongs to the class of substituted salicylic acid derivatives. Structurally, it is a methyl ester of 4-(acetylamino)-2-hydroxybenzoic acid, featuring a hydroxy group ortho to a carboxylic acid moiety (in its esterified form) and an acetylated amino group para to the hydroxy group on the aromatic ring.
The molecule can be described as having a salicylate core with modifications that influence both its physicochemical properties and potential bioactivity. The hydroxy group at position 2 (ortho to the ester) allows for potential intramolecular hydrogen bonding, which can affect the compound’s conformation and solubility in various solvents. Meanwhile, the acetylamino group at position 4 introduces a polar, hydrogen bond-accepting and -donating substituent, which can play a role in intermolecular interactions.
This compound can be synthesized via esterification of the corresponding acid derivative or through directed acetylation and methylation of precursor compounds like 4-amino-2-hydroxybenzoic acid. The methyl ester functionality improves its lipophilicity and stability, making it suitable for certain synthetic or biological applications where a free acid would be less desirable due to potential reactivity or solubility limitations.
In pharmaceutical and biochemical research, derivatives of hydroxybenzoic acids, particularly those with amino or acylated amino substituents, are often explored for anti-inflammatory, analgesic, antimicrobial, or enzyme-inhibitory properties. While specific biological data for methyl 4-(acetylamino)-2-hydroxybenzoate may not be extensively cataloged, its core structure shares similarity with other bioactive molecules, such as paracetamol (acetaminophen) analogs and non-steroidal anti-inflammatory agents, which makes it of potential interest in medicinal chemistry.
The ester group in this molecule is susceptible to hydrolysis under acidic or basic conditions, allowing for the regeneration of the carboxylic acid form. This characteristic can be exploited in prodrug design, where the ester is used to enhance bioavailability and the active acid is released in vivo. Additionally, the presence of both hydrogen bond donors and acceptors can influence the compound’s interaction with biological targets, including enzymes and receptors.
Analytical characterization of methyl 4-(acetylamino)-2-hydroxybenzoate typically involves techniques such as nuclear magnetic resonance (NMR) spectroscopy, infrared (IR) spectroscopy, mass spectrometry (MS), and high-performance liquid chromatography (HPLC). The compound shows characteristic IR absorption bands due to the ester (C=O stretch around 1730 cm⁻¹), acetylamide (around 1650 cm⁻¹), and phenolic O–H (broad band around 3200–3500 cm⁻¹). In NMR spectra, the aromatic region reveals distinct splitting patterns, while the acetyl methyl group and ester methyl protons appear as singlets in the aliphatic region.
In terms of applications, this compound could serve as an intermediate in organic synthesis or as a scaffold in the design of more complex molecules. The functional groups provide multiple handles for further derivatization, including amidation, acylation, or coupling reactions with other aromatic or aliphatic substituents. As such, it is useful in the preparation of pharmaceuticals, dyes, or materials where aromatic esters and amides are integral.
Safety considerations for handling this compound include typical precautions for esters and acylated amines. It should be stored in a cool, dry place away from oxidizers and acids. Personal protective equipment should be used to avoid inhalation or skin contact during handling.
Overall, methyl 4-(acetylamino)-2-hydroxybenzoate represents a chemically versatile compound with potential uses in research and development across chemistry and pharmaceutical science.
References
2008. Design, synthesis, inhibitory activity, and SAR studies of hydrophobic p-aminosalicylic acid derivatives as neuraminidase inhibitors. Bioorganic & Medicinal Chemistry, 16(7).
DOI: 10.1016/j.bmc.2008.01.036
2021. Non-carbohydrate strategies to inhibit lectin proteins with special emphasis on galectins. European Journal of Medicinal Chemistry, 224.
DOI: 10.1016/j.ejmech.2021.113561
|



















 GHS07 Warning Details
GHS07 Warning Details