Online Database of Chemicals from Around the World
| Shanghai 3s Technology Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (021) 5290-7809 | |||
 |
market@3s-tech.net | |||
| Chemical manufacturer since 2019 | ||||
| chemBlink standard supplier since 2025 | ||||
| Classification | Chemical reagent >> Organic reagent >> Thiol ester |
|---|---|
| Name | 2(N-(4,5-dimethyl-isoxazol-3-yl)-N-methoxymethyl-benzenesulfonamide)-boronic acid pinakol ester |
| Synonyms | N-(4,5-dimethyl-1,2-oxazol-3-yl)-N-(methoxymethyl)-2-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzenesulfonamide |
| Molecular Structure | 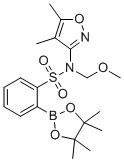 |
| Molecular Formula | C19H27BN2O6S |
| Molecular Weight | 422.30 |
| CAS Registry Number | 415697-56-2 |
| EC Number | 871-102-8 |
| SMILES | B1(OC(C(O1)(C)C)(C)C)C2=CC=CC=C2S(=O)(=O)N(COC)C3=NOC(=C3C)C |
| Hazard Symbols |
 GHS09 Details
GHS09 Details | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard Statements | H411 Details | ||||||||||||
| Precautionary Statements | P273-P391-P501 Details | ||||||||||||
| Hazard Classification | |||||||||||||
| |||||||||||||
|
The chemical substance 2-(N-(4,5-dimethyl-isoxazol-3-yl)-N-methoxymethyl-benzenesulfonamide)-boronic acid pinacol ester is a complex, functionalized aromatic compound featuring a sulfonamide-linked isoxazole, a methoxymethyl protecting group, and a boronic acid pinacol ester. It is valued as a synthetic intermediate, particularly in pharmaceutical and materials chemistry. Its discovery and applications are well-documented in the literature, rooted in the development of sulfonamide chemistry, isoxazole derivatives, and organoboron reagents. The origins of this compound are tied to the study of sulfonamides, which have been explored since the early 20th century for their antibacterial properties and utility as synthetic intermediates. Isoxazoles, five-membered heterocycles containing oxygen and nitrogen, gained prominence in the mid-20th century for their presence in bioactive molecules. The boronic acid pinacol ester, specifically the 4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl group, became a key functional group in the 1980s and 1990s with the advent of Suzuki-Miyaura cross-coupling reactions, pioneered by Akira Suzuki. The methoxymethyl (MOM) protecting group, used to mask sulfonamide nitrogens, was popularized in the 1970s for its stability and ease of removal. The combination of these elements—a sulfonamide bridging a 4,5-dimethylisoxazole and a benzene ring with a boronic ester and MOM group—emerged in the late 20th century to meet the pharmaceutical industry’s demand for versatile, multifunctional intermediates. Synthetically, 2-(N-(4,5-dimethyl-isoxazol-3-yl)-N-methoxymethyl-benzenesulfonamide)-boronic acid pinacol ester is prepared through a multi-step process. A typical route starts with 2-bromobenzenesulfonyl chloride, which is reacted with 4,5-dimethylisoxazol-3-amine to form the sulfonamide. The sulfonamide nitrogen is protected with a methoxymethyl group using chloromethyl methyl ether under basic conditions. The bromine at the 2-position of the benzene ring is then converted to a boronic acid pinacol ester via palladium-catalyzed borylation with bis(pinacolato)diboron or pinacolborane, using a base and a phosphine ligand. Alternatively, the isoxazole can be pre-functionalized and coupled to a sulfonyl chloride already bearing the boronic ester. These steps rely on well-established protocols in sulfonamide synthesis, protecting group chemistry, and organoboron chemistry, ensuring regioselectivity and high yields. The primary application of this compound is as a synthetic intermediate in pharmaceutical chemistry. The isoxazole ring is a privileged scaffold in drugs targeting inflammation, cancer, and infectious diseases, due to its stability and ability to engage in hydrogen bonding. The sulfonamide linkage enhances polarity and serves as a bioisostere, improving pharmacokinetic properties. The boronic acid pinacol ester is a key handle for Suzuki-Miyaura cross-coupling reactions, enabling the introduction of aryl, alkenyl, or alkynyl groups to the benzene ring. The methoxymethyl group protects the sulfonamide nitrogen, allowing selective transformations, and can be removed under acidic conditions to yield the free sulfonamide. This compound is frequently used in the synthesis of kinase inhibitors, receptor modulators, and anti-inflammatory agents, where the combination of heterocyclic, sulfonamide, and boronic ester functionalities optimizes target affinity and drug performance. In materials chemistry, the compound is employed to synthesize functionalized polymers or ligands, where the boronic ester facilitates cross-coupling to build complex architectures. In academic research, it serves as a model for studying cross-coupling mechanisms, sulfonamide reactivity, and the effects of MOM protection. Its synthesis has contributed to advancements in borylation and isoxazole chemistry. The significance of 2-(N-(4,5-dimethyl-isoxazol-3-yl)-N-methoxymethyl-benzenesulfonamide)-boronic acid pinacol ester lies in its role as a multifunctional intermediate that combines the biological relevance of isoxazoles and sulfonamides with the synthetic versatility of boronic esters and MOM protection. Its development reflects progress in heterocyclic synthesis, organoboron chemistry, and protecting group strategies. By enabling the efficient synthesis of complex, biologically active molecules, it has become a critical tool in advancing pharmaceutical, materials, and chemical research. |
| Market Analysis Reports |
| List of Reports Available for 2(N-(4,5-dimethyl-isoxazol-3-yl)-N-methoxymethyl-benzenesulfonamide)-boronic acid pinakol ester |