Online Database of Chemicals from Around the World
| Shijiazhuang C-element Biotech Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 18033710157 | |||
 |
Lisa@C-elementBiotech.com | |||
 |
QQ chat | |||
 |
WeChat: 18033710157 | |||
| Chemical manufacturer since 2025 | ||||
| chemBlink standard supplier since 2025 | ||||
| Classification | Organic raw materials >> Carboxylic compounds and derivatives |
|---|---|
| Name | Diacetyl-L-tartaric acid |
| Synonyms | (2R,3R)-2,3-diacetyloxybutanedioic acid |
| Molecular Structure | 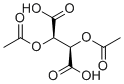 |
| Molecular Formula | C8H10O8 |
| Molecular Weight | 234.16 |
| CAS Registry Number | 51591-38-9 |
| EC Number | 257-303-8 |
| SMILES | CC(=O)O[C@H]([C@H](C(=O)O)OC(=O)C)C(=O)O |
| Density | 1.5±0.1 g/cm3 Calc.* |
|---|---|
| Boiling point | 398.9±42.0 ºC 760 mmHg (Calc.)* |
| Flash point | 159.4±21.4 ºC (Calc.)* |
| Index of refraction | 1.492 (Calc.)* |
| * | Calculated using Advanced Chemistry Development (ACD/Labs) Software. |
| Hazard Symbols |
 GHS07 Warning Details
GHS07 Warning Details | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard Statements | H315-H319 Details | ||||||||||||||||
| Precautionary Statements | P264-P264+P265-P280-P302+P352-P305+P351+P338-P321-P332+P317-P337+P317-P362+P364 Details | ||||||||||||||||
| Hazard Classification | |||||||||||||||||
| |||||||||||||||||
| SDS | Available | ||||||||||||||||
|
Diacetyl-L-tartaric acid is a derivative of L-tartaric acid, a naturally occurring organic compound known for its significant role in various biochemical and industrial applications. L-tartaric acid itself was first isolated from wine sediments in the 18th century and has long been studied for its optical activity and stereochemistry. The discovery of L-tartaric acid’s ability to rotate plane-polarized light led to foundational developments in stereochemistry, particularly through the work of Louis Pasteur in the mid-19th century. Diacetyl-L-tartaric acid is obtained through the chemical modification of L-tartaric acid by esterifying its two hydroxyl groups with acetyl groups. The preparation of diacetyl-L-tartaric acid involves reacting L-tartaric acid with acetic anhydride under controlled conditions, resulting in the substitution of the hydroxyl groups with acetyl functionalities. This modification reduces the hydrophilicity of the molecule compared to the parent compound and influences its chemical reactivity and solubility properties. The chemical structure retains the two carboxyl groups of tartaric acid, while the hydroxyl groups are converted into acetate esters, altering the molecule’s ability to participate in hydrogen bonding. The primary application of diacetyl-L-tartaric acid is in the chemical and pharmaceutical industries as an intermediate or reagent. It is used particularly in the preparation of chiral compounds and enantiomeric resolution processes. The diacetyl derivative provides advantages in certain synthesis protocols where controlling the polarity and reactivity of tartaric acid is beneficial. By masking the hydroxyl groups, chemists can direct reactions more selectively or prevent unwanted side reactions that might otherwise occur if free hydroxyl groups were present. In addition to its use in synthesis, derivatives of diacetyl-L-tartaric acid, such as diacetyl-L-tartaric acid salts, have found utility as chiral resolving agents. In this role, they facilitate the separation of racemic mixtures into their individual enantiomers by forming diastereomeric salts, which can then be separated based on differences in solubility or crystallization behavior. Such processes are critical in the pharmaceutical industry, where the biological activity of drug enantiomers can differ significantly. Diacetyl-L-tartaric acid also has applications in food and beverage technology. Although the parent L-tartaric acid is more commonly used directly as an acidulant and stabilizer, certain modified tartaric acid derivatives, including esters like diacetyl-L-tartaric acid, have been explored for specific functional uses, particularly in modifying texture or stability in food systems. However, their usage is much less widespread compared to simpler tartaric acid salts such as potassium bitartrate. The compound’s chemical properties, including its relatively increased lipophilicity compared to L-tartaric acid, make it useful in organic solvent systems where unmodified tartaric acid would have limited solubility. This characteristic expands the range of chemical environments in which tartaric acid's stereochemical control can be employed. Furthermore, the esterification also imparts increased thermal stability, allowing the compound to be used under reaction conditions where L-tartaric acid might degrade. In the broader context of organic synthesis, diacetyl-L-tartaric acid is a representative example of how simple biochemical building blocks can be chemically modified to create a range of functional intermediates. Its development and use illustrate the ongoing importance of chiral compounds and their derivatives in modern chemical industries. The precise stereochemical properties and the ability to fine-tune reactivity through functional group modification continue to drive interest in substances like diacetyl-L-tartaric acid. There have been no significant reports of diacetyl-L-tartaric acid exhibiting biological activity on its own, and it is generally handled as a chemical reagent rather than a therapeutic agent or direct additive. Safety data indicate that it should be handled with typical precautions appropriate for organic acid derivatives, particularly due to the presence of reactive ester groups. In summary, diacetyl-L-tartaric acid, derived from L-tartaric acid, is an important chemical intermediate, particularly valued for its role in chiral synthesis and resolution. Its preparation and applications reflect fundamental principles of organic and stereochemistry, showcasing the continued relevance of modified natural products in scientific and industrial contexts. |
| Market Analysis Reports |
| List of Reports Available for Diacetyl-L-tartaric acid |