Copper(I) bromide-dimethyl sulfide, often abbreviated as CuBr·DMS, is a significant coordination complex with various applications in organic synthesis and catalysis. The compound features copper(I) bromide coordinated with dimethyl sulfide (DMS), a combination that has proven useful in a range of chemical processes. The discovery and applications of Copper(I) bromide-dimethyl sulfide illustrate its role in advancing both synthetic chemistry and industrial applications.
The synthesis of Copper(I) bromide-dimethyl sulfide involves the coordination of dimethyl sulfide to Copper(I) bromide. The reaction typically occurs in an inert atmosphere to prevent oxidation of the copper(I) center. This complex is prepared by mixing Copper(I) bromide with dimethyl sulfide under controlled conditions, leading to the formation of a stable coordination compound. The dimethyl sulfide acts as a ligand that stabilizes the copper(I) center, enhancing its utility in various catalytic reactions.
Structurally, Copper(I) bromide-dimethyl sulfide consists of a Copper(I) ion coordinated to a bromide ion and dimethyl sulfide ligand. The copper(I) ion, which is in the +1 oxidation state, is a key component in many catalytic processes due to its ability to facilitate electron transfer and participate in oxidation-reduction reactions. The dimethyl sulfide ligand stabilizes the copper(I) center and influences its reactivity.
One of the primary applications of Copper(I) bromide-dimethyl sulfide is in organic synthesis, particularly in coupling reactions. The compound is used as a catalyst in various cross-coupling reactions, such as the Ullmann coupling and the Sonogashira coupling. In these reactions, Copper(I) bromide-dimethyl sulfide facilitates the formation of carbon-carbon bonds by mediating the transfer of electrons between reactants. This application is crucial for the synthesis of complex organic molecules, including pharmaceuticals and fine chemicals.
In addition to coupling reactions, Copper(I) bromide-dimethyl sulfide is employed in other catalytic processes. For example, it is used in the oxidation of alcohols to aldehydes or ketones. The compound's ability to participate in electron transfer processes makes it a valuable catalyst for these transformations, which are important in the synthesis of various organic compounds.
Copper(I) bromide-dimethyl sulfide also finds applications in the field of material science. The compound is used in the preparation of organic semiconductors and light-emitting materials. Its role as a catalyst in the synthesis of these materials contributes to the development of advanced electronic devices and displays.
The stability and reactivity of Copper(I) bromide-dimethyl sulfide make it a useful tool in both laboratory and industrial settings. The compound should be handled with care to avoid exposure to air and moisture, which can lead to the oxidation of the copper(I) center and degradation of the complex. Proper storage in a dry, inert atmosphere is essential to maintain its effectiveness.
Future research on Copper(I) bromide-dimethyl sulfide may focus on improving its catalytic efficiency and exploring new applications. Advances in understanding its coordination chemistry and reactivity could lead to the development of more effective catalysts and new synthetic methodologies.
|



















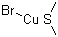
 GHS07 Warning Details
GHS07 Warning Details